解毒化瘀通腑方对乙型肝炎肝硬化肝胆湿热证患者肠道菌群的影响
DOI: 10.3969/j.issn.1001-5256.2022.04.016
Effect of Jiedu Huayu Tongfu prescription on intestinal flora in patients with hepatitis B cirrhosis with liver-gallbladder damp-heat syndrome
-
摘要:
目的 研究解毒化瘀通腑方对乙型肝炎肝硬化肝胆湿热证患者肠道稳态的调节作用及对内毒素、炎性因子和细胞免疫功能的影响。 方法 纳入2019年6月至2021年1月在河南中医药大学第一附属医院就诊,符合诊断及纳入标准的72例患者为研究对象,随机分为观察组和对照组,每组各36例。其治疗组脱落2例,剔除2例,完成32例;对照组脱落2例,剔除1例,完成33例。两组均给予抗病毒、保肝等基础治疗,观察组加服解毒化瘀通腑颗粒,对照组给予双歧杆菌四联活菌片口服,疗程为4周。运用16S rDNA测序技术对两组粪便菌群进行基因测序,同时检测治疗前后肝功能(ALT、AST、TBil、Alb)、内毒素(ET)、TNFα、IL-6水平以及T淋巴细胞亚群(CD3+T、CD4+T、CD8+T、CD4+/CD8+)的变化。计量资料服从正态分布及方差齐性时,组内比较采用配对t检验,2组间比较用独立样本t检验,不服从正态分布时用Wilcoxon秩和检验;计数资料组间比较采用χ2检验。 结果 观察组临床总有效率为87.5%,对照组总有效率为60.6%,观察组总有效率明显高于对照组(χ2=-2.299,P=0.022)。两组治疗后ALT、AST、TBil均降低,Alb水平升高(P值均<0.05),与对照组相比,观察组TBil水平降低更显著(Z=-2.165,P=0.030)。两组患者治疗后CD3+T、CD4+T、CD4+/CD8+水平均明显改善, 且观察组较对照组更显著(Z值分别为-2.146、-2.940、3.157,P值分别为0.032、0.003、0.002)。两组患者治疗后炎性因子TNFα、IL-6与ET水平均明显下降,且观察组优于对照组(Z值分别为-2.139、-1.982、-2.062,P值分别为0.032、0.048、0.043)。两组治疗后OTU数量均较前上升。肠道菌群结构丰度在门水平上,观察组治疗后厚壁菌门丰度的上升和拟杆菌门丰度的下降较治疗前更显著(Z值分别为-3.181、-2.215,P值分别为0.001、0.027);与对照组相比,观察组厚壁菌门、蓝细菌门丰度的升高和拟杆菌门、梭菌门、ε-变形菌门的降低更明显(P值均<0.05);在属水平上,观察组双歧杆菌属较治疗前升高更明显(Z=-2.045,P=0.041)。Alpha多样性分析表明,观察组Chao1、Ace指数较治疗前升高更加显著(t值分别为-4.263、-3.328,P值分别为0.001、0.005),其中Ace指数较对照组上升更明显(t=2.292,P=0.030)。Beta多样性分析表明,各组菌群构成相似,无明显统计学差异(P值均>0.05)。 结论 在病因及基础治疗的基础上联合解毒化瘀通腑方治疗能够缓解乙型肝炎肝硬化肝胆湿热证患者临床症状,减轻肝损伤,改善细胞免疫功能。解毒化瘀通腑方可能通过增加厚壁菌门、乳杆菌属、双歧杆菌属等有益菌的丰度和减少拟杆菌门、梭菌门等致病菌的丰度来改善患者肠道菌群失调,其对肝脏和免疫功能的进一步改善作用可能与调整肠道微生态有关。 Abstract:Objective To investigate the regulatory effect of Jiedu Huayu Tongfu prescription on intestinal homeostasis in patients with hepatitis B cirrhosis with liver-gallbladder damp-heat syndrome, as well as its effect on endotoxin, inflammatory factors, and cellular immune function. Methods A total of 72 patients who attended The First Affiliated Hospital of Henan University of Chinese Medicine from June 2019 to January 2021 and met the diagnostic and inclusion criteria were enrolled as subjects and then randomly divided into observation group and control group, with 36 patients in each group. In the treatment group, 2 patients were lost to follow-up, 2 patients were excluded, and 32 patients completed the study; in the control group, 2 patients were lost to follow-up, 1 patient was excluded, and 33 patients completed the study. In addition to the basic treatment including antiviral therapy and liver-protecting treatment, the patients in the observation group were given Jiedu Huayu Tongfu granules, and those in the control group were given oral administration of Bifidobacterium tetravaccine tablets; the course of treatment was 4 weeks for both groups. The 16S rDNA sequencing technique was used for sequencing of fecal flora, and the two groups were measured in terms of the changes in liver function [alanine aminotransferase (ALT), aspartate aminotransferase (AST), total bilirubin (TBil), and albumin (Alb)], endotoxin (ET), levels of tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6), and T lymphocyte subsets (CD3+T, CD4+T, CD8+T, and CD4+/CD8+) after treatment. For normally distributed continuous data with homogeneity of variance, the paired t-test was used for comparison within each group, and the independent samples t-test was used for comparison between two groups; the Wilcoxon rank-sum test was used for comparison of non-normally distributed continuous data. The chi-square test was used for comparison of categorical data. Results The observation group had a significantly higher overall response rate than the control group (87.5% vs 60.6%, χ2=-2.299, P=0.022). After treatment, both groups had significant reductions in ALT, AST, and TBil and a significant increase in Alb (all P < 0.05), and compared with the control group, the observation group had a significantly greater reduction in TBil (Z=-2.165, P=0.030). After treatment, both groups had significant improvements in the levels of CD3+T, CD4+T, and CD4+/CD8+, and the observation group had significantly greater improvements than the control group (Z=-2.146, -2.940, and 3.157, P=0.032, 0.003, and 0.002). After treatment, both groups had significant reductions in the levels of TNF-α, IL-6, and ET, and the observation group had significantly greater reductions than the control group (Z=-2.139, -1.982, and -2.062, P=0.032, 0.048, and 0.043). Both groups had an increase in the number of operational taxonomic units after treatment. As for the abundance of intestinal flora at the phylum level, the observation group had a significant increase in the abundance of Firmicutes and a significant reduction in the abundance of Bacteroidetes after treatment (Z=-3.181 and -2.215, P=0.001 and 0.027); compared with the control group, the observation group had significantly greater increases in the abundance of Firmicutes and Cyanobacteria and significantly greater reductions in the abundance of Bacteroidetes, Cercozoa, and ε-Proteobacteria (allP < 0.05). At the genus level, the observation group had a significant increase in the abundance of Bifidobacterium after treatment (Z=-2.045, P=0.041). The alpha-diversity analysis showed that the observation group had significant increases in Chao1 and Ace indices after treatment (t=-4.263 and -3.328, P=0.001 and 0.005) and a significantly greater increase in Ace index than the control group (t=2.292, P=0.030). The beta-diversity analysis showed that the two groups had a similar composition of flora without significant difference (all P > 0.05). Conclusion Jiedu Huayu Tongfu prescription, in combination with etiological and basic treatments, can alleviate clinical symptoms, reduce liver injury, and improve cellular immune function in patients with hepatitis B cirrhosis with liver-gallbladder damp-heat syndrome. Jiedu Huayu Tongfu prescription can improve the imbalance of intestinal flora by increasing the abundance of the probiotic bacteria such as Firmicutes, Lactobacillus, and Bifidobacterium and the pathogenic bacteria such as Bacteroidetes and Cercozoa, and its effect in further improving liver and immune function may be associated with the regulation of intestinal microecology. -
肝硬化是临床常见的慢性、进行性、弥漫性的终末期肝病。HBV感染是导致肝硬化发生发展的主要原因。随着"肠-肝轴"理论的提出, 越来越多研究[1-2]显示肠道菌群失调在肝硬化及其并发症的发生发展中具有重要的影响。前期研究[3]表明, 解毒化瘀通腑方可改善酒精性肝硬化及肝衰竭患者肝功能, 降低内毒素水平, 其作用机制可能与影响TLR4/NF-κB通路炎性级联信号的传导, 抑制NF-κB的激活, 减少炎性递质及细胞因子的释放有关。肝硬化及肝衰竭时肠道菌群紊乱和疾病的进展关系密切, 解毒化瘀通腑方能否改善乙型肝炎肝硬化患者肠道微生态失衡?其对肝脏的保护作用是否与调整肠道微生态有关尚缺乏进一步研究。故本研究将通过观察解毒化瘀通腑方对乙型肝炎肝硬化肝胆湿热证患者肠道菌群的调节作用和对内毒素、炎性因子水平、细胞免疫功能的影响, 为中医药防治肝硬化肠道菌群紊乱和临床治疗肠道微生态失衡提供更多依据和方法。
1. 资料与方法
1.1 研究对象
纳入2019年6月至2021年1月在河南中医药大学第一附属医院接受治疗, 符合纳入及排除标准的门诊及住院患者72例, 其中观察组2例未按疗程治疗剔除, 2例中途退出, 完成32例; 对照组2例中途退出, 1例未按疗程治疗, 完成33例; 总脱落率为9.7%。所纳入患者诊断符合中华医学会《慢性乙型肝炎防治指南(2019年版)》[4]关于乙型肝炎肝硬化的诊断标准; 符合中华中医药学会肝胆病分会制订的《病毒性肝炎中医辨证标准》(2017年版)[5]关于肝胆湿热证的证候诊断标准。纳入标准: (1)年龄18~70岁; (2)肝功能分级Child-Pugh A/B级。排除标准: (1)2周内服用过任何种类益生菌制剂及免疫调节剂者; (2)合并全身或局部感染、合并大量腹水、合并肝性脑病、消化道出血等严重并发症者; (3)合并其他引起肝硬化的因素如HCV、饮酒或药物等; (4)合并肿瘤, 严重心、脑、肾、造血系统疾病, 内分泌、风湿和精神病患者; (5)孕妇和哺乳期妇女。
1.2 治疗方法
观察组: 根据病情在抗病毒、保肝降酶及对症治疗基础上, 给予解毒化瘀通腑颗粒剂口服: 茵陈30 g、黄芩15 g、赤芍30 g、金钱草30 g、郁金15 g、大黄10 g。上述颗粒剂由本院药房(四川新绿色)统一提供, 1副/d, 上午及下午分2次温开水冲服。连续服用5 d, 休息2 d。对照组: 根据病情在抗病毒、保肝降酶及对症治疗基础上, 给予双歧杆菌四联活菌片(思连康)口服(杭州远大生物制药有限公司, 国药准字S20060010, 规格: 每片0.5 g), 3片/次, 3次/d。治疗时间为4周, 观察期间均规律饮食, 保持相对稳定的饮食结构。
1.3 观察指标
治疗前后, 采用全自动生化检测仪检测肝功能(ALT、AST、TBil、Alb); 采用流式细胞仪检测T淋巴细胞亚群(CD3+、CD4+、CD8+、CD4+/CD8+); 采集入组患者清晨空腹肘静脉血4 mL, 离心, 分离血清, 采用酶联免疫吸附法(ELISA)检测血清TNFα、IL-6、ET水平。
1.4 菌群检测及分析
采集受试者治疗前后晨起新鲜粪便样本, 置于-80℃超低温冰箱内保存待测。采集完成的标本进行16S rDNA测序分析。包括粪便DNA提取、DNA质量检测、文库构建与质检, 采用MiSeq平台2×250 bp的双端测序策略对文库进行测序, 得到最终分析所使用的有效序列, 进行相似度>97%的OTU聚类分析并计算其丰度。基于OTU聚类结果, 进行Alpha和Beta多样性分析得到不同样本和分组之间的群落结构差异。利用Metastats软件对组间的物种丰度数据进行t检验, 对分组样品的物种组成进行组间显著性分析。
1.5 疗效评价标准
参考《中药新药临床研究指导原则(试行)》[6]中的中医证候分级量化标准进行评分, 临床症状: 胁肋胀痛、身目发黄、食少纳呆、口干口苦、尿黄。按无、轻度、中度、重度分别记为0、2、4、6分。显效: 症状、体征明显改善, 证候积分减少≥ 70%;有效: 症状、体征均有好转, 证候积分减少≥ 30%;无效: 症状、体征均无明显改善, 甚或加重, 证候积分减少不足30%。积分变化公式(尼莫地平法): [(治疗前症状总积分-治疗后症状总积分)/治疗前症状总积分]×100%。
1.6 统计学方法
采用SPSS 25.0进行数据分析。符合正态分布的计量资料以x±s表示, 组内比较采用配对t检验, 2组间比较用独立样本t检验; 非正态分布计量资料以M(P25~P75)表示, 组间比较采用Wilcoxon秩和检验; 计数资料组间比较采用χ2检验。P < 0.05为差异有统计学意义。
2. 结果
2.1 两组患者一般资料及中医症状疗效比较
观察组中男21例, 女11例; 年龄29~67岁, 平均(48.50±8.92)岁; 肝功能Child-Pugh分级: A级18例、B级14例; 对照组中男22例, 女11例; 年龄34~68岁, 平均(52.24±9.20)岁; 肝功能分级: A级20例、B级13例; 两组患者性别、年龄、肝功能分级比较差异均无统计学意义(P值均>0.05), 具有可比性。
观察组总有效率87.5%, 对照组总有效率为60.6%, 观察组总有效率明显高于对照组(χ2=-2.299, P=0.022)(表 1)。
表 1 两组中医症状疗效比较Table 1. Comparison of curative effects of TCM symptoms between the two groups组别 例数 显效[例(%)] 有效[例(%)] 无效[例(%)] 总有效率(%) 观察组 32 7(21.9) 21(65.6) 4(12.5) 87.5 对照组 33 4(12.1) 16(48.5) 13(39.4) 60.6 2.2 两组治疗前后肝功能比较
两组治疗前ALT、AST、TBil、Alb比较差异均无统计学意义(P值均>0.05);与治疗前相比, 两组治疗后ALT、AST、TBil均有不同程度的降低(P值均 < 0.05), Alb升高(P < 0.05);与对照组相比, 观察组治疗后TBil水平下降更加显著(P=0.030)(表 2)。
表 2 两组肝功能比较Table 2. Comparison of liver function between the two groups组别 例数 ALT(U/L) AST(U/L) 治疗前 治疗后 Z值 P值 治疗前 治疗后 Z值 P值 观察组 32 44.45(36.78~66.38) 32.50(27.45~44.08) 7.007 < 0.001 48.85(33.15~63.33) 37.40(29.93~42.53) -4.507 < 0.001 对照组 33 47.50(33.75~65.30) 33.80(24.80~49.55) 5.599 < 0.001 46.20(33.40~65.00) 35.10(26.85~50.25) -4.083 < 0.001 Z值 -0.046 -0.387 -0.092 -0.013 P值 0.963 0.699 0.927 0.990 组别 例数 TBil(μmol/L) Alb(g/L) 治疗前 治疗后 Z值 P值 治疗前 治疗后 Z值 P值 观察组 32 20.08(17.25~39.75) 15.80(12.85~21.23) -4.506 < 0.001 35.50(30.35~41.83) 38.25(34.60~43.78) -2.940 0.006 对照组 33 25.20(16.30~38.45) 23.70(15.10~33.00) -2.207 0.027 35.20(29.95~40.05) 36.20(33.50~40.00) -2.472 0.019 Z值 -0.020 -2.165 0.326 1.760 P值 0.984 0.030 0.745 0.083 2.3 两组治疗前后T淋巴细胞亚群水平比较
两组治疗前CD3+T、CD4+T、CD8+T、CD4+/CD8+比较差异均无统计学意义(P值均>0.05);与治疗前相比, 两组CD3+T、CD4+T、CD4+/CD8+均有不同程度的升高(P值均 < 0.05), 观察组CD8+T淋巴细胞显著降低(P < 0.05);与对照组相比, 观察组治疗后CD3+T、CD4+T、CD4+/CD8+升高更明显(P值均 < 0.05)(表 3)。
表 3 两组T淋巴细胞亚群水平比较Table 3. Comparison of T lymphocyte subsets between the two groups组别 例数 CD3+T(%) CD4+T(%) 治疗前 治疗后 Z值 P值 治疗前 治疗后 Z值 P值 观察组 32 61.10(49.73~68.80) 71.55(67.60~74.25) -5.189 < 0.001 32.05(24.48~40.28) 43.20(38.70~46.15) -5.321 < 0.001 对照组 33 61.70(48.90~68.70) 68.20(60.35~71.10) -6.745 < 0.001 33.70(25.85~39.95) 38.00(32.85~41.45) -2.537 0.011 Z值 -0.066 -2.146 -0.217 -2.940 P值 0.948 0.032 0.829 0.003 组别 例数 CD8+T(%) CD4+/CD8+ 治疗前 治疗后 Z值 P值 治疗前 治疗后 Z值 P值 观察组 32 26.40(22.00~36.75) 25.55(20.65~28.43) 2.565 0.015 1.27(0.92~1.52) 1.69(1.55~1.93) -6.983 < 0.001 对照组 33 29.70(24.25~36.25) 27.70(23.50~30.90) 1.788 0.083 1.05(0.72~1.59) 1.43(1.06~1.80) -2.381 0.023 Z值 -0.513 -1.887 -0.741 3.157 P值 0.610 0.064 0.458 0.002 2.4 两组治疗前后炎症指标比较
两组治疗前IL-6、TNFα、ET比较均无统计学差异(P值均>0.05);与治疗前相比, 两组治疗后IL-6、TNFα、ET均有不同程度的下降(P值均 < 0.05);与对照组相比, 观察组IL-6、TNFα、ET下降水平更明显(P值均 < 0.05)(表 4)。
表 4 两组治疗前后炎症指标比较Table 4. Comparison of inflammatory indexes between the two groups组别 例数 IL-6(pg/mL) TNFα(pg/mL) ET(pg/mL) 治疗前 治疗后 治疗前 治疗后 治疗前 治疗后 观察组 32 5.04(4.05~6.00) 2.73(1.77~3.16) 57.59(42.37~67.27) 26.94(16.20~42.59) 9.28(7.49~11.41) 6.18(4.00~8.20) 对照组 33 4.99(4.27~6.18) 3.05(2.51~3.90) 58.38(32.30~71.68) 48.18(15.69~65.41) 10.05(8.59~12.16) 8.12(5.48~9.90) Z值 -0.518 -2.139 -0.039 -1.982 -0.688 -2.062 P值 0.604 0.032 0.969 0.048 0.494 0.043 2.5 两组治疗前后肠道菌群分析
2.5.1 OUT结果分析
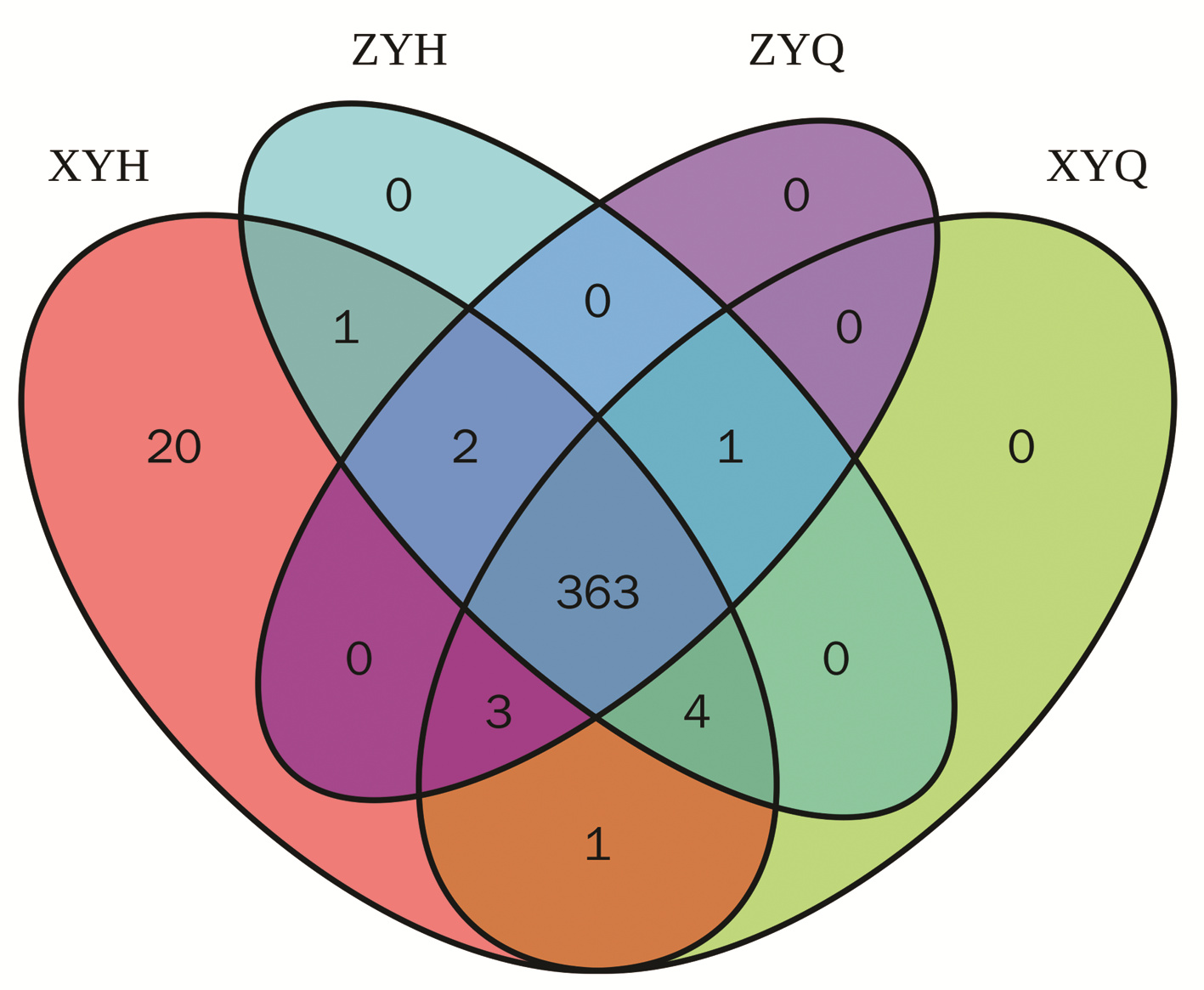
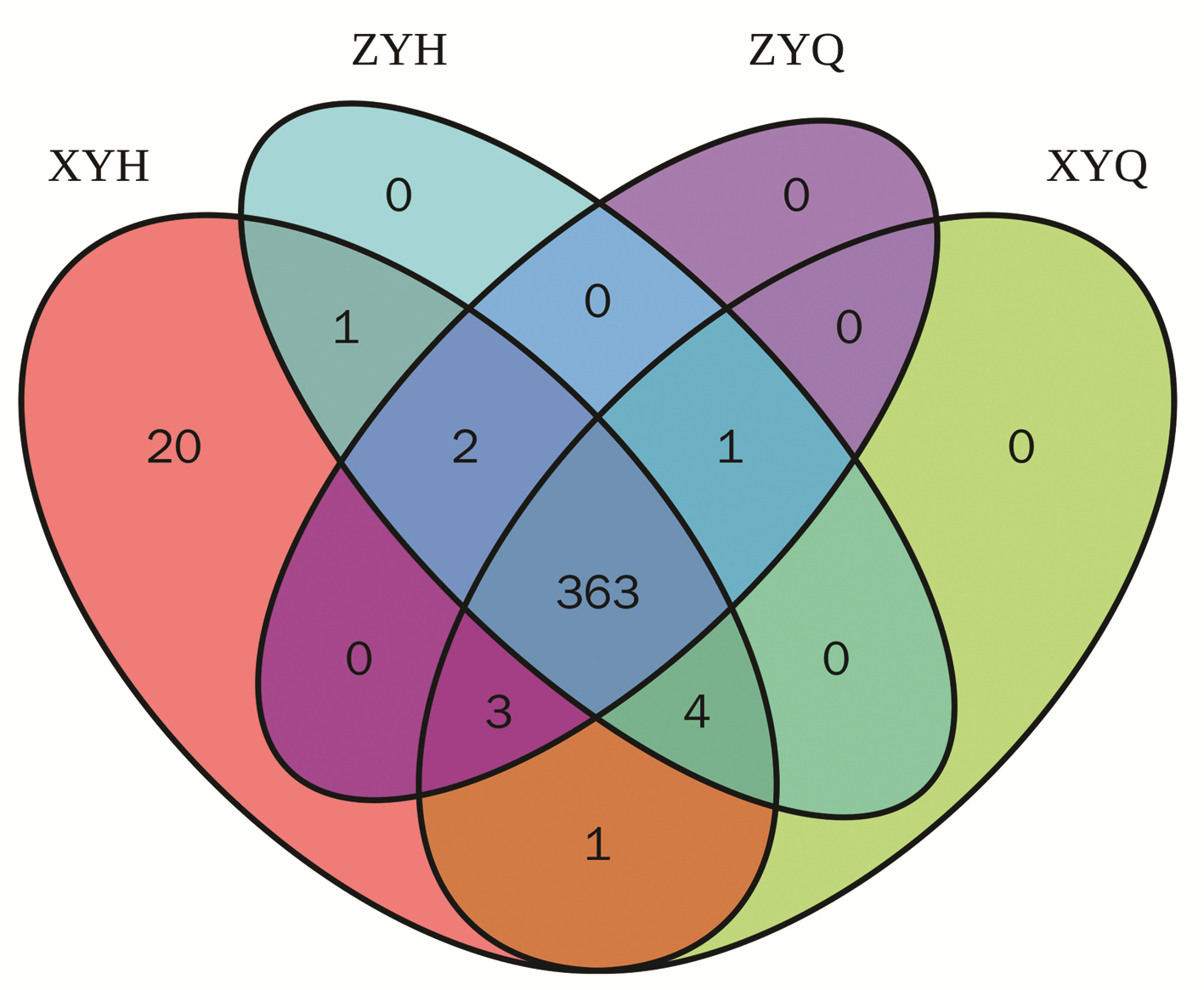
对获得的样本进行测序分析, 共获得6 145 843个原始数据, 然后对双端测序数据进行拼接、质控过滤及去除嵌合体后得到443 0889条有效序列, 平均每个样品73 848条, Tag平均长度为418 bp。使用usearch软件对有效序列进行聚类(软件默认序列相似性高于97%的划分为一个OTU), 共有的OTU为363个。观察组和对照组治疗前OTU个数分别为372和369个, 治疗后分别为394和371个, 两组治疗后OTU数量均较前上升(图 1)。
2.5.2 两组肠道菌群结构丰度分析
2.5.2.1 肠道菌群在门水平上的分析
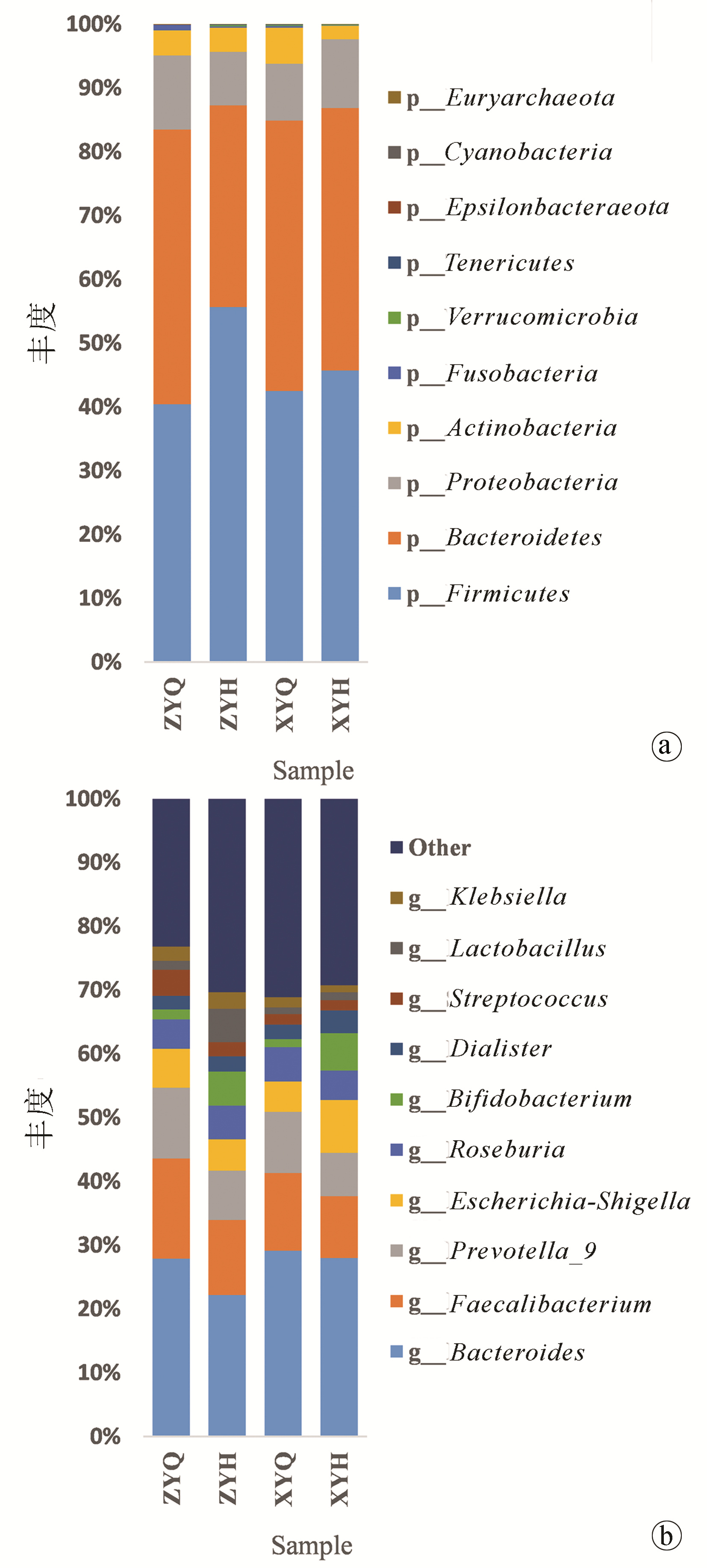
在门(Phylum)水平上相对丰度前十的菌门有厚壁菌门(Firmicutes)、拟杆菌门(Bacteroidetes)、变形菌门(Proteobacteria)、放线菌门(Actinobacteria)、梭菌门(Fusobacteria)、疣微菌门(Verrucomicrobia)、软壁菌门(Tenericutes)、ε-变形菌门(Epsilonbacteraeota)、蓝细菌门(Cyanobacteria)和广古菌门(Euryarchaeota)。其中厚壁菌门、拟杆菌门和变形菌门为三个优势菌门。与治疗前相比, 观察组治疗后厚壁菌门丰度上升和拟杆菌门下降显著(Z值分别为-3.181、-2.215, P值分别为0.001、0.027);与对照组相比, 观察组厚壁菌门、蓝细菌门丰度升高显著, 拟杆菌门、梭菌门、ε-变形菌门降低更明显(Z值分别为-2.800、-2.177、-2.077、-2.509、-2.373, P值分别为0.005、0.029、0.047、0.012、0.018)(图 2a)。
2.5.2.2 肠道菌群在属水平上的分析
在属(Genus)水平上, 拟杆菌属(Bacteroides)、粪杆菌属(Faecalibacterium)、普雷沃菌属(Prevotella_9)、埃希菌-志贺菌属(Escherichia-Shigella)、罗氏菌属(Roseburia)、双歧杆菌属(Bifidobacterium)、戴阿利斯特菌属(Dialister)、乳杆菌属(Lactobacillus)、链球菌属(Streptococcus)、克雷伯氏菌属(Klebsiella)为丰度排名前十的物种, 其中拟杆菌属、粪杆菌属、普雷沃菌属和链球菌属治疗后均较治疗前有下降趋势, 双歧杆图菌属、戴阿利斯特菌属和乳杆菌属较治疗前有升高趋势, 其中观察组双歧杆菌属治疗后较治疗前升高更显著(Z=-2.045, P=0.041)(图 2b)。
2.5.3 Alpha多样性分析
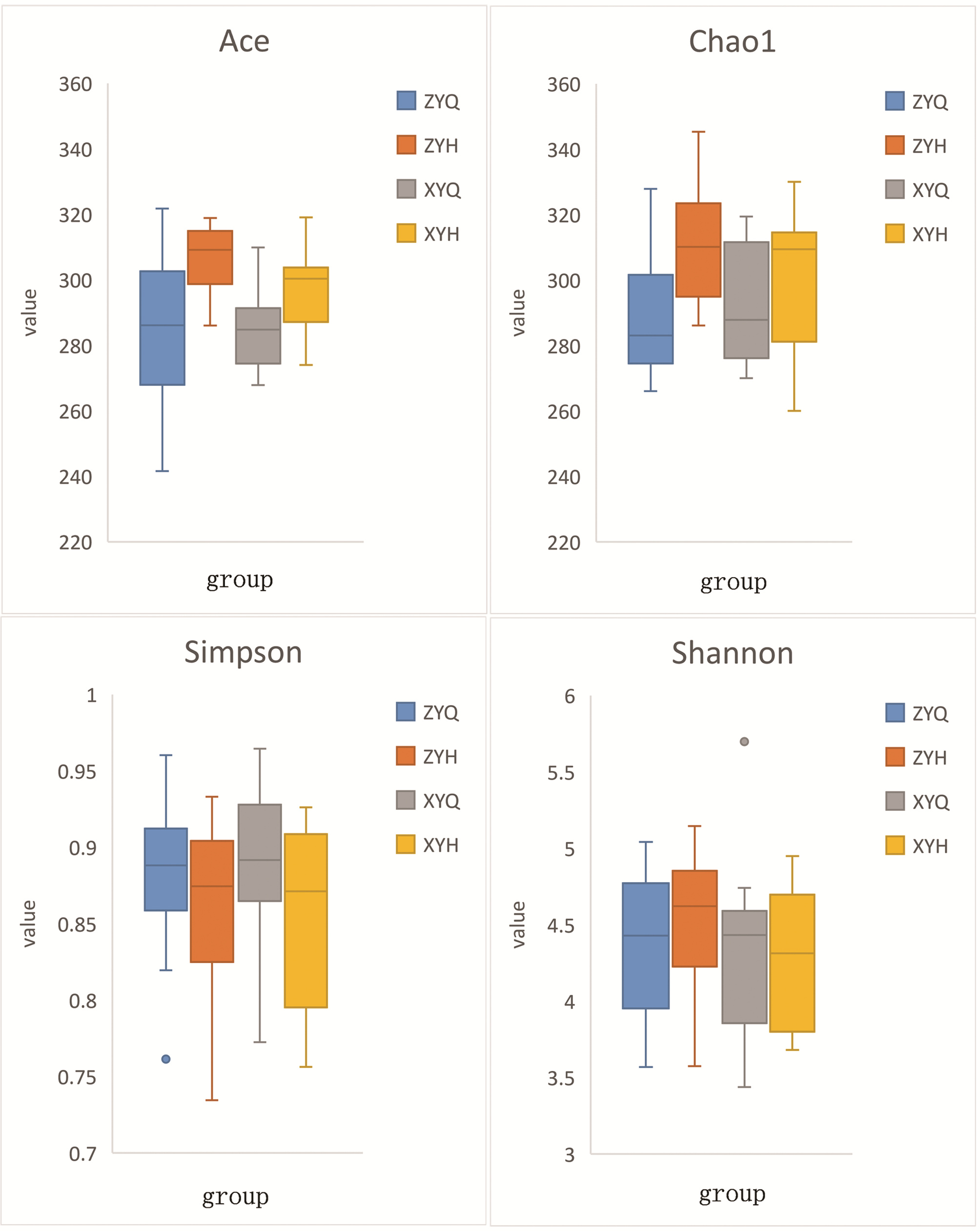
Alpha多样性是指一个特定区域或者系统内的多样性, 反映的是单个样品物种丰度及物种多样性。Chao1和Ace指数用来计算菌群的丰度, 值越大代表物种总数越多。Shannon和Simpson指数用来计算菌群的多样性, 相同物种丰度的情况下, Shannon指数值越大, Simpson指数值越小, 样品的物种多样性越高。本研究显示, 与治疗前相比, 观察组Chao1、Ace指数均明显升高(t值分别为-4.263、-3.328, P值分别为0.001、0.005), Shannon、Simpson指数无明显统计学差异(P值均>0.05);与对照组比较, 观察组治疗后Ace指数上升更明显(t=2.292, P=0.030), 余指数无明显统计学差异(P值均>0.05), 故观察组所含物种总数更多, 但对肠道菌群多样性方面没有明显影响(图 3)。
2.5.4 Beta多样性分析
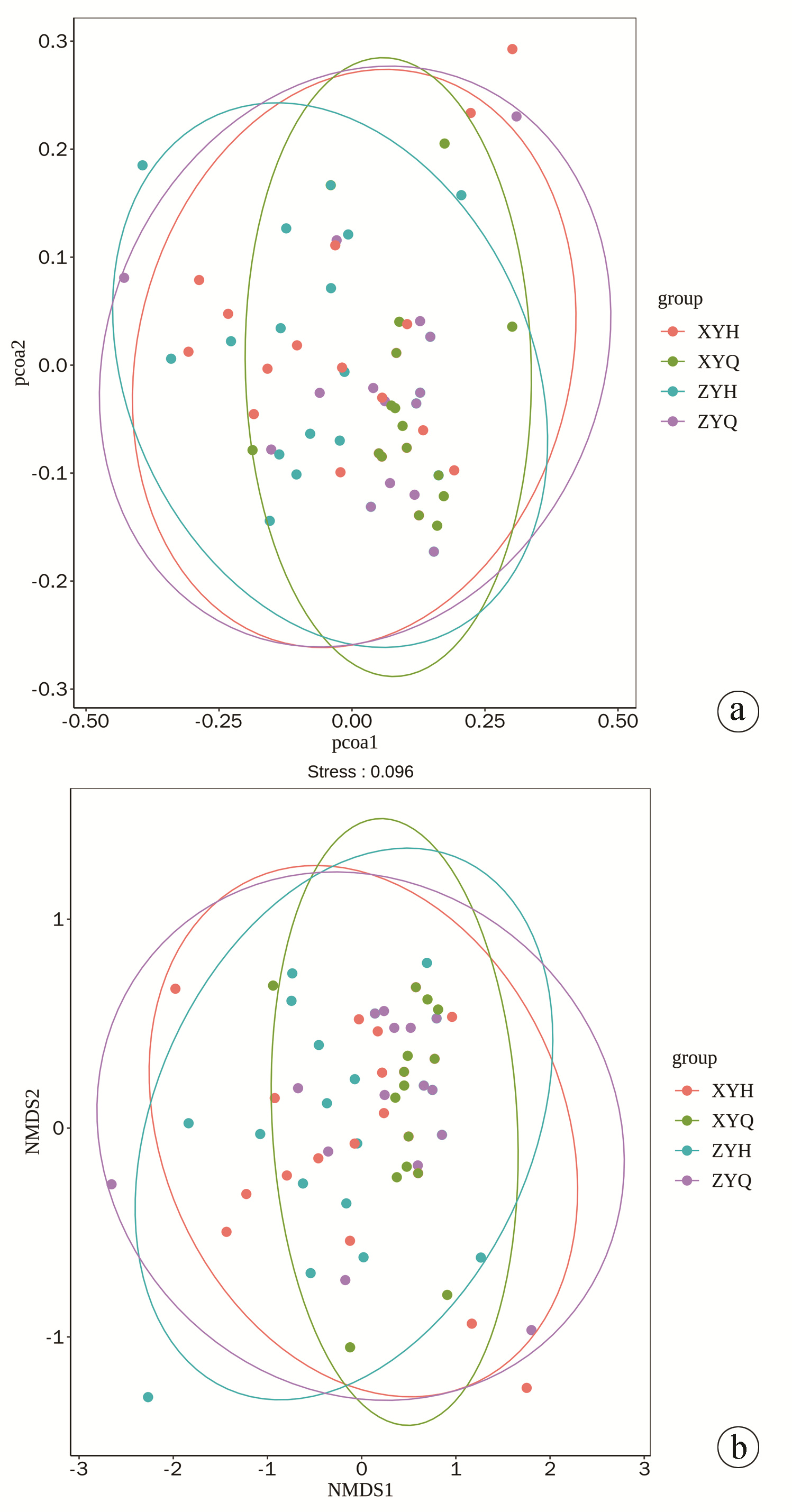
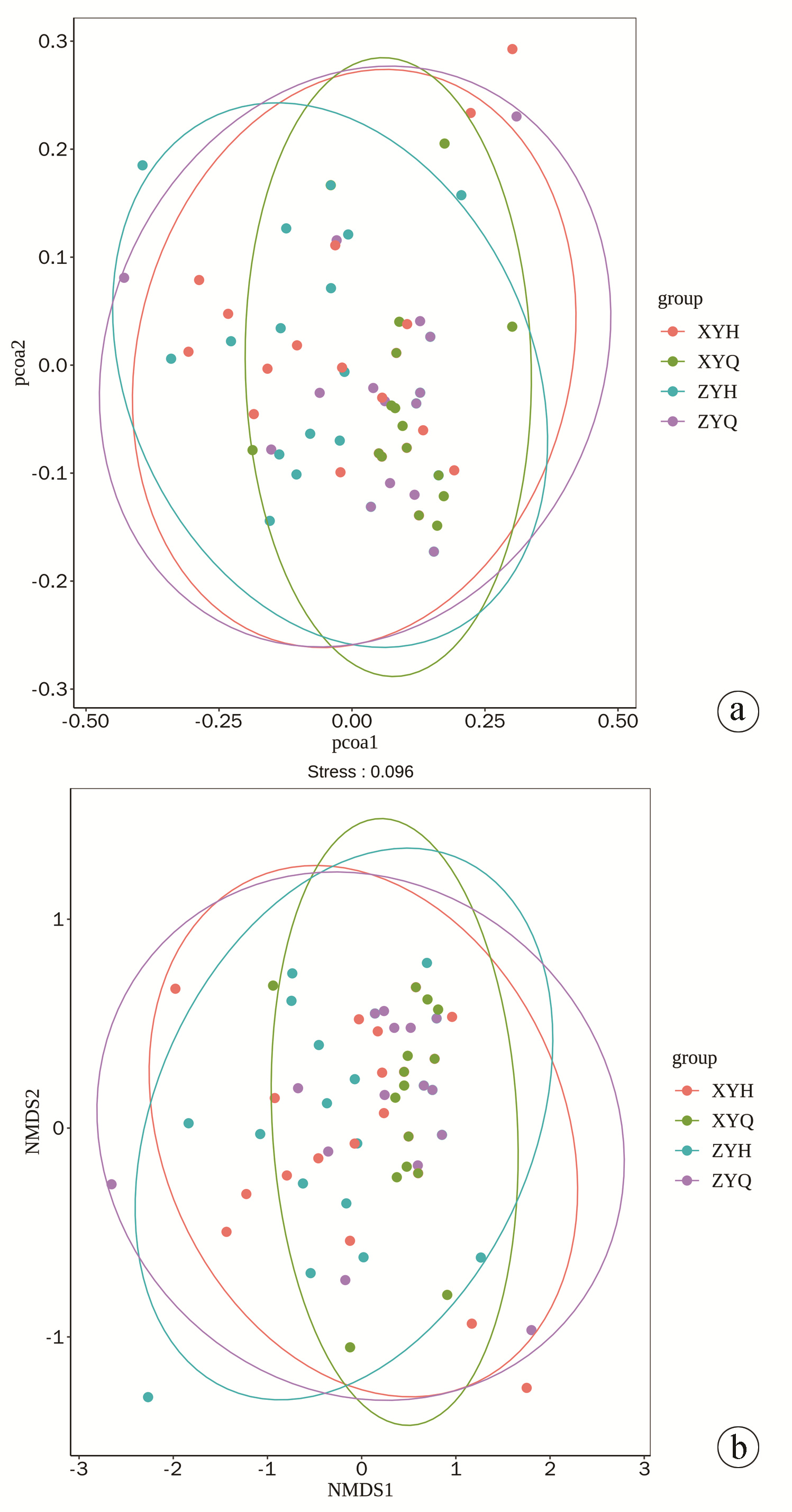
使用Qiime软件进行Beta多样性分析, 比较不同样品在物种多样性方面存在的相似程度。本研究中, 基于Weighted Unifrac算法绘制PCoA和NMDS分析结果图, 坐标图上样品距离越近, 则表示相似性越大。由图可见, 两组治疗后与治疗前出现轻度分类聚集现象, 但未达到显著水平。Anosim分析结果表明, 组间菌群差异无明显统计学意义(P=0.743), 各组样品物种构成相似(图 4)。
3. 讨论
肝硬化是以肝组织纤维化和正常肝结构转化为肝硬化结节为特征的慢性、进行性的终末期肝病。肠道菌群与肝脏主要通过肠-肝轴在解剖学和功能上密切联系。肝硬化患者小肠细菌的过度生长和肠道通透性的增加, 导致肝性脑病、自发性细菌性腹膜炎、门静脉高压症、肝肾综合征和多脏器衰竭等与细菌或内毒素移位相关的肝硬化肠源性并发症的发生在临床已有广泛的报道[7]。肝硬化与肠道菌群失调相互影响, 互为因果, 往往形成恶性循环。故调节肠道菌群, 维持肠道微生态平衡对防治肝硬化有重要的意义。目前常见的治疗方法主要有微生态制剂的应用, 如益生菌、益生元、合生元等, 以及抗生素、粪菌移植等方法[8]。但均存在一定的局限性, 临床应用效果不甚满意。
中医学将乙型肝炎肝硬化归为"胁痛""积聚""臌胀"等范畴, HBV属湿热疫毒之邪, 作为肝硬化的重要病理因素, 贯穿疾病发生发展的始终。本病病机变化往往沿着湿、热、毒、瘀、虚的动态变化而不断发展, 病位多涉及肝胆、脾胃, 机体因情志不遂或感受湿热、疫毒之邪等导致肝脏失于疏泄、调达, 脾胃运化功能失司, 水湿停聚中焦而不得化, 加之疫毒之邪助湿生热, 日久形成瘀滞之势, 临床则表现出胁肋胀痛、口干口苦、腹胀、脘闷、纳呆、困重等症状。湿热之邪蕴于中焦, 而"六腑以通为用", 需得"中结者使之旁达", 给湿热瘀浊之邪以去路。故肝胆湿热证作为该病发展的早期阶段, 治疗上需"清热解毒、化瘀通腑"。解毒化瘀通腑方由茵陈、黄芩、赤芍、金钱草、郁金、大黄组成, 方中茵陈为苦寒下降, 清利湿热毒邪之君药; 黄芩清热燥湿, 助茵陈解毒退黄, 金钱草清热利胆, 除湿退黄, 与黄芩共为臣药; 郁金行气活血、凉血逐瘀; 赤芍清热凉血祛瘀, 助郁金祛湿退黄, 郁金功专肝胆二经, 能引诸药直达病所, 兼起使药之功; 佐以大黄泄热除瘀, 通利大便。纵观全方, 诸药合用, 共奏解毒化瘀通腑之功效。
在肝硬化中, 肠道微生物群的改变可能是诱导和维持肠道炎症、肠屏障破坏和微生物物质向肝板层和邻近器官移位的关键因素, 从而加剧了肝硬化存在的肝脏和全身炎症, 这可能是疾病进展的一个关键因素[9]。IL-6、IL-8、TNFα等作为T淋巴细胞介导分泌的炎症因子, 能够反映机体的免疫状态, 加重炎症反应进程, 损害肝细胞功能[10]。研究[11]发现, 乙型肝炎肝硬化患者ALT、AST、ALP、TNFα、IL-6及IL-8水平与双歧杆菌、乳酸菌等有益菌水平呈负相关, 与肠杆菌科、肠球菌等条件致病菌水平呈正相关。本研究中, 治疗后两组TBil、IL-6、TNFα、ET水平均较治疗前改善, 且观察组优于对照组。肝硬化相关的免疫功能障碍与细菌易位密切联系。肝硬化时, 与细菌易位相关的脾淋巴组织扩张、炎症和脾脏隔离会导致T淋巴细胞数量减少[12]。此外, 过度活化的T淋巴细胞和肠系膜淋巴结的炎症又会促进细菌移位, 最终发展为全身性炎症综合征[13]。McGovern等[14]研究发现, 大多数肝硬化患者往往出现T淋巴细胞计数的下降, 主要表现为CD4+T淋巴细胞缺乏症。在本研究中, 治疗后两组患者CD3+T、CD4+T、CD4+/CD8+淋巴细胞百分比均较治疗前上升, 且观察组较对照组改善更显著, 表明解毒化瘀通腑方能改善肝硬化患者细胞免疫功能。
与健康人相比, 肝硬化患者存在肠道菌群失调, 表现为部分潜在致病菌的过度生长和有益菌的减少, 增加感染风险[15]。肝硬化患者的OTU数量较健康人群显著下降, 肠道菌群丰度及多样性随肝硬化程度的加重而降低[16]。肠道菌群失调与肝硬化患者病死率的增加密切相关。Zeng等[17]研究发现, 慢性乙型肝炎患者的厚壁菌门丰度低于健康组, 拟杆菌门丰度高于健康组, 且在肝硬化与肝癌组中亦有类似发现。研究表明[18-19], 厚壁菌门/拟杆菌门的比值被广泛认为对维持正常的肠道内环境稳定有重要影响, 其比值的降低往往表现为炎症性疾病。本研究中, 观察组治疗后出现更显著的厚壁菌门、蓝细菌门比例的升高和拟杆菌门、梭菌门、ε-变形菌门比例的降低。IL-6、TNFα等促炎因子的含量与变形菌门的丰度呈正相关, 与厚壁菌门等保护性细菌呈负相关[20]。同时经Alpha和Beta多样性分析发现两组治疗后菌群丰度及多样性有所改善, 但两组差异不明显。有证据表明蓝细菌与非酒精性肝病的死亡风险呈显著正相关, 但蓝细菌门在肝硬化中研究较少, 尚不明确其作用机制[21]。
综上, 在病因及基础治疗的基础上联合解毒化瘀通腑方治疗能够缓解乙型肝炎肝硬化肝胆湿热证患者临床症状, 减轻肝损伤, 改善细胞免疫功能。解毒化瘀通腑方可能通过增加厚壁菌门、乳杆菌属、双歧杆菌属等有益菌的丰度和减少拟杆菌门、梭菌门等致病菌的丰度来改善患者肠道菌群失调, 其对肝脏和免疫功能的进一步改善作用可能与调整肠道微生态有关。
-
表 1 两组中医症状疗效比较
Table 1. Comparison of curative effects of TCM symptoms between the two groups
组别 例数 显效[例(%)] 有效[例(%)] 无效[例(%)] 总有效率(%) 观察组 32 7(21.9) 21(65.6) 4(12.5) 87.5 对照组 33 4(12.1) 16(48.5) 13(39.4) 60.6 表 2 两组肝功能比较
Table 2. Comparison of liver function between the two groups
组别 例数 ALT(U/L) AST(U/L) 治疗前 治疗后 Z值 P值 治疗前 治疗后 Z值 P值 观察组 32 44.45(36.78~66.38) 32.50(27.45~44.08) 7.007 < 0.001 48.85(33.15~63.33) 37.40(29.93~42.53) -4.507 < 0.001 对照组 33 47.50(33.75~65.30) 33.80(24.80~49.55) 5.599 < 0.001 46.20(33.40~65.00) 35.10(26.85~50.25) -4.083 < 0.001 Z值 -0.046 -0.387 -0.092 -0.013 P值 0.963 0.699 0.927 0.990 组别 例数 TBil(μmol/L) Alb(g/L) 治疗前 治疗后 Z值 P值 治疗前 治疗后 Z值 P值 观察组 32 20.08(17.25~39.75) 15.80(12.85~21.23) -4.506 < 0.001 35.50(30.35~41.83) 38.25(34.60~43.78) -2.940 0.006 对照组 33 25.20(16.30~38.45) 23.70(15.10~33.00) -2.207 0.027 35.20(29.95~40.05) 36.20(33.50~40.00) -2.472 0.019 Z值 -0.020 -2.165 0.326 1.760 P值 0.984 0.030 0.745 0.083 表 3 两组T淋巴细胞亚群水平比较
Table 3. Comparison of T lymphocyte subsets between the two groups
组别 例数 CD3+T(%) CD4+T(%) 治疗前 治疗后 Z值 P值 治疗前 治疗后 Z值 P值 观察组 32 61.10(49.73~68.80) 71.55(67.60~74.25) -5.189 < 0.001 32.05(24.48~40.28) 43.20(38.70~46.15) -5.321 < 0.001 对照组 33 61.70(48.90~68.70) 68.20(60.35~71.10) -6.745 < 0.001 33.70(25.85~39.95) 38.00(32.85~41.45) -2.537 0.011 Z值 -0.066 -2.146 -0.217 -2.940 P值 0.948 0.032 0.829 0.003 组别 例数 CD8+T(%) CD4+/CD8+ 治疗前 治疗后 Z值 P值 治疗前 治疗后 Z值 P值 观察组 32 26.40(22.00~36.75) 25.55(20.65~28.43) 2.565 0.015 1.27(0.92~1.52) 1.69(1.55~1.93) -6.983 < 0.001 对照组 33 29.70(24.25~36.25) 27.70(23.50~30.90) 1.788 0.083 1.05(0.72~1.59) 1.43(1.06~1.80) -2.381 0.023 Z值 -0.513 -1.887 -0.741 3.157 P值 0.610 0.064 0.458 0.002 表 4 两组治疗前后炎症指标比较
Table 4. Comparison of inflammatory indexes between the two groups
组别 例数 IL-6(pg/mL) TNFα(pg/mL) ET(pg/mL) 治疗前 治疗后 治疗前 治疗后 治疗前 治疗后 观察组 32 5.04(4.05~6.00) 2.73(1.77~3.16) 57.59(42.37~67.27) 26.94(16.20~42.59) 9.28(7.49~11.41) 6.18(4.00~8.20) 对照组 33 4.99(4.27~6.18) 3.05(2.51~3.90) 58.38(32.30~71.68) 48.18(15.69~65.41) 10.05(8.59~12.16) 8.12(5.48~9.90) Z值 -0.518 -2.139 -0.039 -1.982 -0.688 -2.062 P值 0.604 0.032 0.969 0.048 0.494 0.043 -
[1] SANTIAGO A, POZUELO M, POCA M, et al. Alteration of the serum microbiome composition in cirrhotic patients with ascites[J]. Sci Rep, 2016, 6: 25001. DOI: 10.1038/srep25001. [2] MUÑOZ L, BORRERO MJ, U ' BEDA M, et al. Intestinal immune dysregulation driven by dysbiosis promotes barrier disruption and bacterial translocation in rats with cirrhosis[J]. Hepatology, 2019, 70(3): 925-938. DOI: 10.1002/hep.30349. [3] LIU JK, ZHAO WX, LIU QH, et al. Effect of Jiedu Huayu Tongfu Recipe on endotoxin and inflammatory factors in patients with alcoholic liver cirrhosis[J]. China J Tradit Chin Med Pharma, 2017, 32(12): 5668-5671. https://www.cnki.com.cn/Article/CJFDTOTAL-BXYY201712115.htm刘江凯, 赵文霞, 刘巧红, 等. 解毒化瘀通腑方对酒精性肝硬化患者内毒素及炎性因子的影响[J]. 中华中医药杂志, 2017, 32(12): 5668-5671. https://www.cnki.com.cn/Article/CJFDTOTAL-BXYY201712115.htm [4] Chinese Society of Infectious Diseases, Chinese Medical Association, Chinese Society of Hepatology, Chinese Medical Association. Guidelines for the prevention and treatment of chronic hepatitis B (version 2019)[J]. J Clin Hepatol, 2019, 35(12): 2648-2669. DOI: 10.3969/j.issn.1001-5256.2019.12.007.中华医学会感染病学分会, 中华医学会肝病学分会. 慢性乙型肝炎防治指南(2019年版)[J]. 临床肝胆病杂志, 2019, 35(12): 2648-2669. DOI: 10.3969/j.issn.1001-5256.2019.12.007. [5] Branch of Hepatobiliary Diseases, Chinese Association of Chinese Medicine. The standards of traditional Chinese medicine syndrome differentiation for viral hepatitis[J]. J Clin Hepatol, 2017, 33(10): 1839-1846. DOI: 10.3969/j.issn.1001-5256.2017.10.002.中华中医药学会肝胆病分会. 病毒性肝炎中医辨证标准[J]. 临床肝胆病杂志, 2017, 33(10): 1839-1846. DOI: 10.3969/j.issn.1001-5256.2017.10.002. [6] ZHENG XY. Guiding principles for clinical research of new traditional Chinese medicine (Trial)[M]. Beijing: China Medical Science and Technology Press, 2002.郑筱萸. 中药新药临床研究指导原则(试行)[M]. 北京: 中国医药科技出版社, 2002. [7] MINEMURA M, SHIMIZU Y. Gut microbiota and liver diseases[J]. World J Gastroenterol, 2015, 21(6): 1691-1702. DOI: 10.3748/wjg.v21.i6.1691. [8] WANG WF, CAO JB, FAN GR, et al. Meta analysis of probiotics in patients with liver cirrhosis[J]. Beijing Med J, 2015, 37 (3): 213-219. DOI: 10.15932/j.0253-9713.2015.3.005.王伟芳, 曹建彪, 范公忍, 等. 益生菌在肝硬化患者应用的Meta分析[J]. 北京医学, 2015, 37(3): 213 -219. DOI: 10.15932/j.0253-9713.2015.3.005. [9] SOLÉ C, GUILLY S, DA SILVA K, et al. Alterations in gut microbiome in cirrhosis as assessed by quantitative metagenomics: Relationship with acute-on-chronic liver failure and prognosis[J]. Gastroenterology, 2021, 160(1): 206-218. e13. DOI: 10.1053/j.gastro.2020.08.054. [10] MARTINS M, BATISTA A, BRITO Y, et al. Effect of remote ischemic preconditioning on systemic toxicity and ototoxicity induced by cisplatin in rats: Role of TNF-α and Nitric Oxide[J]. ORL J Otorhinolaryngol Relat Spec, 2017, 79(6): 336-346. DOI: 10.1159/000485514. [11] ZHANG W, PENG Q. Relationship between intestinal flora and liver function and serum inflammatory factors in patients with hepatitis B cirrhosis[J]. Mod Digest Interv, 2020, 25(2): 158-162. DOI: 10.3969/j.issn.1672-2159.2020.02.006.张雯, 彭琼. 乙肝肝硬化患者肠道菌群与肝功能及血清炎症因子水平的关系[J]. 现代消化及介入诊疗, 2020, 25(2): 158-162. DOI: 10.3969/j.issn.1672-2159.2020.02.006. [12] LARIO M, MUÑOZ L, UBEDA M, et al. Defective thymopoiesis and poor peripheral homeostatic replenishment of T-helper cells cause T-cell lymphopenia in cirrhosis[J]. J Hepatol, 2013, 59(4): 723-730. DOI: 10.1016/j.jhep.2013.05.042. [13] MUNOZ L, ALBILLOS A, NIETO M, et al. Mesenteric Th1 polarization and monocyte TNF-alpha production: First steps to systemic inflammation in rats with cirrhosis[J]. Hepatology, 2005, 42(2): 411-419. [14] MCGOVERN BH, GOLAN Y, LOPEZ M, et al. The impact of cirrhosis on CD4+ T cell counts in HIV-seronegative patients[J]. Clin Infect Dis, 2007, 44(3): 431-437. DOI: 10.1086/509580. [15] ZENG Y, CHEN S, FU Y, et al. Gut microbiota dysbiosis in patients with hepatitis B virus-induced chronic liver disease covering chronic hepatitis, liver cirrhosis and hepatocellular carcinoma[J]. J Viral Hepat, 2020, 27(2): 143-155. DOI: 10.1111/jvh.13216. [16] GUO XX, HU N, LIAN XX, et al. Features of intestinal flora imbalance in patients with liver cirrhosis and related driving factors[J]. J Clin Hepatol, 2020, 36(7): 1527-1533. DOI: 10.3969/j.issn.1001-5256.2020.07.016.郭晓霞, 胡娜, 廉晓晓, 等. 肝硬化患者肠道菌群失调的特征及驱动因子分析[J]. 临床肝胆病杂志, 2020, 36(7): 1527-1533. DOI: 10.3969/j.issn.1001-5256.2020.07.016. [17] ZENG Y, CHEN S, FU Y, et al. Gut microbiota dysbiosis in patients with hepatitis B virus-induced chronic liver disease covering chronic hepatitis, liver cirrhosis and hepatocellular carcinoma[J]. J Viral Hepat, 2020, 27(2): 143-155. [18] ABENAVOLI L, SCARPELLINI E, COLICA C, et al. Gut microbiota and obesity: A role for probiotics[J]. Nutrients, 2019, 11(11): 2690. DOI: 10.3390/nu11112690. [19] SHEN ZH, ZHU CX, QUAN YS, et al. Relationship between intestinal microbiota and ulcerative colitis: Mechanisms and clinical application of probiotics and fecal microbiota transplantation[J]. World J Gastroenterol, 2018, 24(1): 5-14. DOI: 10.3748/wjg.v24.i1.5. [20] CAO H, WU DS, ZHANG Y, et al. Effects of shaoyao decoction on intestinal flora of ulcerative colitis rats based on high-throughput sequencing technology[J]. Chin J Inf Tradit Chin Med, 2021, 28(1): 61-66. DOI: 10.19879/j.cnki.1005-5304.202006249.曹晖, 吴东升, 张彧, 等. 基于高通量测序技术研究芍药汤对溃疡性结肠炎大鼠肠道菌群的影响[J]. 中国中医药信息杂志, 2021, 28(1): 61-66. DOI: 10.19879/j.cnki.1005-5304.202006249. [21] ZHANG F, LEE J, LIANG S, et al. Cyanobacteria blooms and non-alcoholic liver disease: evidence from a county level ecological study in the United States[J]. Environ Health, 2015, 14: 41. DOI: 10.1186/s12940-015-0026-7. -




 PDF下载 ( 3527 KB)
PDF下载 ( 3527 KB)

 下载:
下载:




 下载:
下载: