中医药调控上皮-间质转化抑制肝癌转移的研究进展
DOI: 10.3969/j.issn.1001-5256.2022.11.040
Research advances in traditional Chinese medicine in regulating epithelial-mesenchymal transformation to inhibit hepatocellular carcinoma metastasis
-
摘要: 转移是影响肝癌复发和死亡的主要原因,而上皮-间质转化(EMT)是肝癌转移的重要机制。EMT受TGFβ、Wnt/β-catenin、Notch等多种信号通路介导的转录因子Snail、Twist、ZEB等的调控。抑制肝癌EMT相关分子和信号通路是抑制肝癌侵袭转移的重要途径。近年来研究表明,多种中药复方或其有效成分具有抑制肝癌侵袭转移的作用,阻滞或逆转肝癌细胞EMT是其重要的作用机制。本文对EMT的作用和调控机制,以及近年来中药及其来源的天然化合物通过调控细胞EMT抑制肝癌侵袭转移的研究进行概述,以期为肝癌转移的中医药防治提供科学依据,为肝癌治疗提供新思路。Abstract: Metastasis is an important factor for the high recurrence and mortality rates of hepatocellular carcinoma (HCC), and epithelial-mesenchymal transition (EMT) is an important mechanism of HCC metastasis. EMT is regulated by the transcription factors such as Snail, Twist, and ZEB which are mediated by a variety of signaling pathways including TGF-β, Wnt/β-catenin, and Notch. Inhibition of EMT-related molecules and signal pathways in HCC is considered as an important approach to inhibit the invasion and metastasis of HCC. Recent studies have shown that a variety of compound traditional Chinese medicine (TCM) formula or their effective constituents can inhibit the invasion and metastasis of HCC by arresting or reversing EMT in HCC. This article reviews the role and mechanism of EMT and recent studies on TCM drugs and their derived natural compounds in inhibiting the invasion and metastasis of HCC by regulating cell EMT, so as to provide a scientific basis for the TCM prevention and treatment of HCC metastasis and new ideas for HCC treatment.
-
肝癌是全球第六大常见恶性肿瘤,全球癌症死亡率排名第三,肝细胞癌(HCC)占75%~85%[1]。转移和复发是影响HCC死亡率的主要因素[2]。流行病学数据显示,HCC最常见转移部位为肝,其次是肺、淋巴结、骨等肝外组织[3]。上皮-间质转化(epithelial-mesenchymal transition, EMT) 在肝癌侵袭、转移过程中发挥关键作用[4-5]。EMT是指上皮表型细胞通过特定程序失去连接和极性转化为具有间质表型细胞的生物学过程,赋予了细胞转移、抗凋亡及免疫逃逸能力,在发育、组织创伤愈合、器官纤维化中起关键作用,并参与癌症进程[6]。Pinzani等[4]提出“逃逸反应”假说来解释肿瘤细胞发生EMT转化:肿瘤细胞对瘤组织缺氧、坏死、炎症和氧自由基增加等不利微环境作出适应性反应,转化为间质表型趋向更适宜环境移动以逃避损伤凋亡。在肝癌侵袭转移时,HCC细胞发生EMT有助于传播扩散。HCC患者无肿瘤复发的生存期与EMT水平负相关,而阻滞EMT可显著抑制HCC细胞的迁移[7]。基于EMT关键分子的预后诊断方法及靶向EMT的治疗策略也成为肝癌转移复发的研究热点。
目前手术、放化疗、靶向和免疫治疗在肝癌治疗中发挥重要作用,但伴随的术后转移复发、耐药、副作用等成为治疗的重大障碍[8]。在我国肝癌综合治疗方案里,中医药被长期运用于肝癌的治疗,尤其是对于中晚期患者;而近年来中药来源的多种活性化合物在肝癌治疗研究中的效应也引起广泛关注[9]。《金匮要略·疟病脉证并治》首次提到徵瘕,以坚硬不移,痛有定处者为徵;聚散无常,推之游移不定,痛无定处者为瘕。据其症状,现代医家将肝癌归为“徵”和“瘕”的范畴。中医药基于肝癌“本虚标实”的病机进行系统性的多层面治疗[10]。近年来研究表明,抑制或逆转HCC细胞EMT是中药发挥治疗肝癌侵袭转移作用的重要途径之一。本文介绍EMT相关机制,并对近年来中药及其来源化合物通过调控EMT抑制肝癌侵袭转移的研究进行概述,以期促进发展基于中医药来源的肝癌转移复发防治新策略。
1. HCC转移相关细胞EMT调控机制
EMT受到包括SNAIL(Snail、Slug)、BHLH (Twist1、Twist2、E12、E47)、ZEB(ZEB1、ZEB2)等在内的EMT转录因子(EMT-inducing transcriptions factors,EMT-TF)的调控,使上皮型细胞丢失E-钙黏蛋白、Occludin等细胞间连接相关分子,获得波形蛋白(Vimentin)、纤维连接蛋白(Fibronectin)、N-钙黏蛋白(N-cadherin)等骨架和极性相关蛋白以及基质金属蛋白酶(matrix metalloproteinases,MMP)等间质型标志物,导致细胞间黏附下降、形态改变、迁移和侵袭能力增强[11]。其中SNAIL是肝癌EMT最主要的诱导者,在高转移的肝癌细胞中高表达,诱导EMT促进肝癌细胞转移[12]。
肝癌细胞EMT-TF表达或活化受多种分子调控,如TGFβ、骨形态发生蛋白(bone morphogenetic protein, BMP)、Smad蛋白、表皮生长因子(epidermal growth factor,EGF)、成纤维细胞生长因子(fibroblast growth factor,FGF)、血小板衍生生长因子(platelet- derived growth factor,PDGF)、低氧诱导因子(hypoxia inducible factor,HIF)、整合素等,通过多种信号通路进行调控[13-18]。
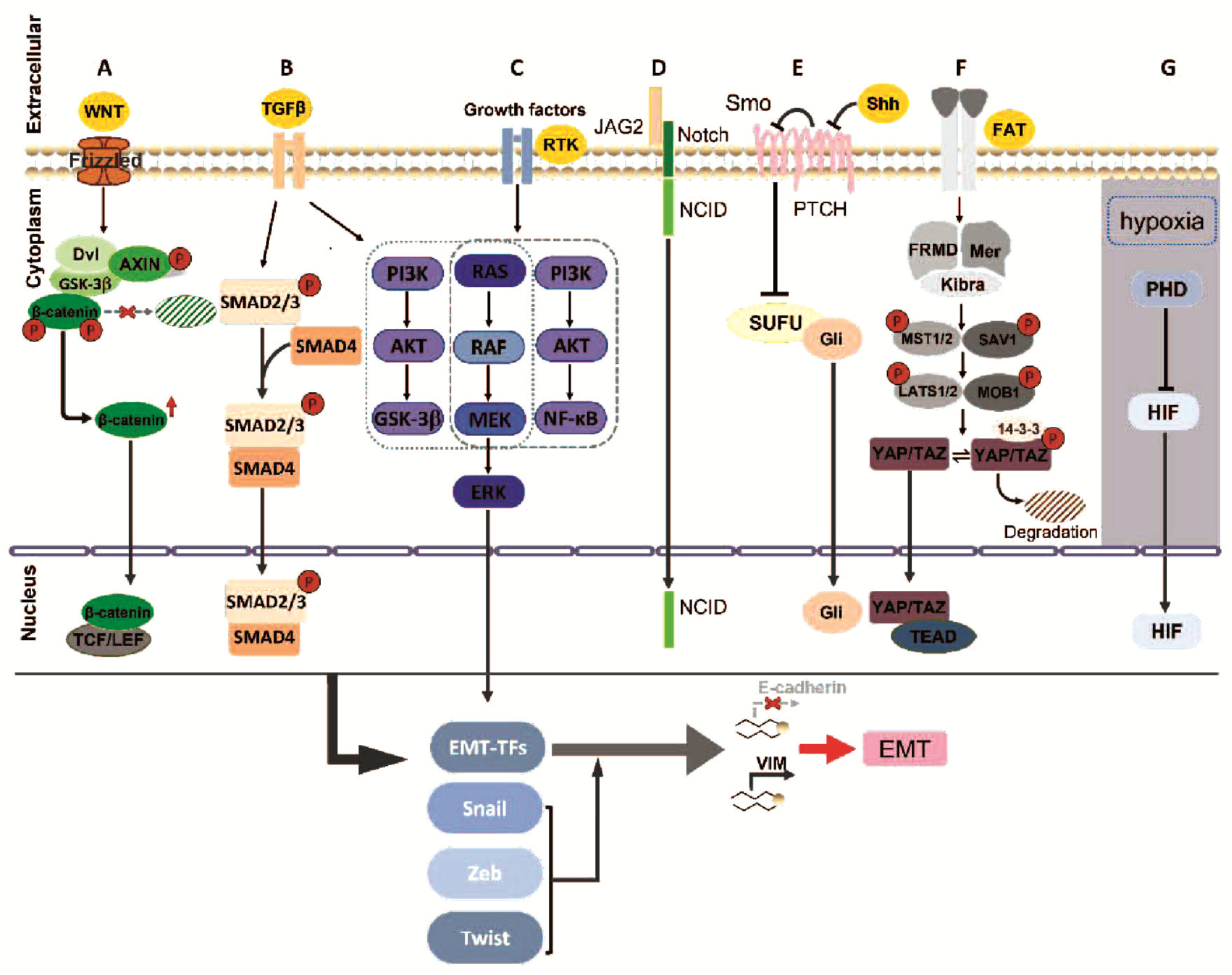
EMT调控信号通路包括TGFβ、Wnt/β-catenin、生长因子(GF)/受体酪氨酸激酶(receptor tyrosine kinases, RTK)、Hedgehog、Notch、Hippo、低氧等信号通路[18](图 1)。TGFβ通路是目前已知介导EMT转化最重要的途径,包括经典途径(Smad依赖通路)和非经典途径(Smad非依赖通路)。Wnt/β-catenin信号通路是EMT调控的另一重要通路,β-catenin入核与TCF/LEF形成复合物,可直接抑制E-钙黏蛋白转录,促进转录因子Snail、Slug、Twist的转录表达。肿瘤微环境中的多种生长因子可结合并激活其相应RTK,继而激活包括由PI3K、Ras、Src和ILK等介导的多种信号通路,激活通路下游EMT转录因子。其他通路也基本通过促进或活化EMT-TF发挥作用。这些信号通路间又存在多个环节的交互调控,如RTK激活的通路也是许多TGFβ诱导的非Smad信号通路,而RTK可通过自分泌信号正反馈回路增强TGFβ1的表达,在EMT转化及肝癌转移过程中共同发挥重要作用[19]。
除基因表达水平外,EMT-TF的转录后水平和功能调控对于EMT也很重要。非编码RNA,尤其是microRNA(miRNA),可直接影响Snail、ZEB1等EMT因子蛋白翻译,此外也可阻断其他信号通路,发挥逆转EMT抑制肿瘤的作用[20],其中EMT相关研究中最多的两类miRNA是miR-34和miR-200家族。长链非编码RNA(long non-coding RNA,lncRNA)在EMT的调节中也发挥重要作用,其中多数参与TGFβ信号调节[21]。
2. 中药或其组分靶向EMT抑制HCC转移的作用和机制
近年来肝癌的中医药干预和个体化治疗体现了其独特优势,中药复方或由中药来源的天然化学物质被认为是HCC的重要补充和替代治疗。其中部分药物机制与EMT调控相关,下面将简介靶向EMT抑制HCC转移的中药或其组分。
2.1 中药复方
临床研究[22]显示,鳖甲煎丸具有缩小肝癌原位瘤生长,抑制复发的作用。钟晓丹等[23]发现鳖甲煎丸含药血清可抑制肝癌细胞HepG2侵袭迁移能力,进一步研究揭示其可抑制NF-κB活化入核阻止Snail的表达,使E-钙黏蛋白表达增加,从而抑制TGFβ诱导的EMT。此外,鳖甲煎丸还能通过抑制GSK-3β的磷酸化促进β-catenin的降解以阻断Wnt/β-catenin信号通路从而逆转EMT[24]。钟崇等[25]通过体外实验发现,健脾化瘀方含药血清可呈剂量依赖性下调肝癌细胞MHCC97H中Smad磷酸化,后者可促进Snail转录进而诱导EMT。冯坤良等[26]发现,健脾化瘀方还可通过调控外泌体从而上调肝癌细胞E-钙黏蛋白表达,抑制N-cadherin和Vimentin表达,降低肝癌细胞侵袭、迁移能力。扶正抗癌方可改善移植性肝癌大鼠生存状态,抑制肿瘤生长转移[27]。方瑜等[28]发现扶正抗癌方含药血清可逆转TGFβ1诱导的HepG2细胞EMT,抑制MMP表达,从而抑制肝癌侵袭转移。梁雪婧等[29]报道软坚护肝片在临床使用中具有预防肝癌复发的作用。肝癌患者术后服用软坚护肝片及甘草甜素片干预较服用葫芦素片及肌苷的对照组患者3年和5年的肝癌复发率均显著下降。孙振等[30]等通过后续实验发现软坚护肝片含药血清能显著逆转TGFβ1诱导的肝癌细胞Huh7发生EMT,从而抑制细胞迁移能力。
中药复方松友饮联合经肝动脉化疗栓塞术在临床治疗中可减轻肝癌肺转移率[31],对其抑制转移复发的效应机制研究较多。动物实验发现,该复方可有效抑制化疗后残余肝癌组织的EMT[32-33],降低残余肝癌组织侵袭转移潜能,并减少残余肝癌再接种的复发及延长裸鼠生存时间。其机制研究表明,松友饮能通过抑制β-catenin通路阻止残余癌组织EMT。此外,松友饮可下调MHCC97H细胞TGFβ表达水平,抑制Smad磷酸化及与Smad4组装成异源三聚体,阻断其入核与相应EMT转录因子结合。中药复方肝积方可抑制肝癌转移复发,提高患者生存率[34]。本课题组近期研究发现,肝积方含药血清可抑制肝癌细胞Akt通路活化及snail、vimentin分子表达,上调其E-钙黏蛋白、N-cadherin表达水平,体内实验同样也显示肝积方对大鼠原位肝癌组织上述EMT相关分子的调控作用,提示肝积方可能通过调控Akt信号通路抑制EMT发挥抑瘤作用。
2.2 单味中药或组分
中药提取物或化合物多年来被认作是药物制备的结构基础,在现代医学肿瘤的新型治疗研究中具有重要地位。近年来,一些具有抑瘤作用的单味中药材或其成分通过体内外实验显示具有逆转EMT作用,其中部分药物与肝癌转移调控机制相关。
程清波等[35]通过侵袭迁移实验发现山慈菇含药血清可显著抑制肝癌细胞HepG2侵袭,其作用与调控EMT转化相关。Yan等[36]通过体内实验发现,全蝎含药血清可以抑制Hepa1-6肝癌细胞的侵袭迁移能力,全蝎水提物能抑制裸鼠模型中肝癌的生长及转移。进一步研究揭示全蝎可逆转肝癌细胞EMT,提高E-钙黏蛋白的表达,降低N-cadherin和Snail的表达。Wang等[37]发现复方苦参注射液干预SMMC7721肝癌细胞,可抑制β-catenin活化导致的c-Myc表达增加,进而调控EMT,减轻细胞侵袭和迁移能力。通过细胞划痕、Transwell实验,付江等[38]发现银杏叶提取物(ginkgo biloba extract, GBE)含药血清中血小板活化因子(PAF)水平降低。用GBE含药血清处理的MHCC97H和Hep3B细胞的增殖、侵袭和迁移能力显著下调,细胞E-钙黏蛋白升高,而Vimentin、Snail表达降低,提示GBE含药血清可能通过下调PAF逆转肝癌细胞EMT。
姜黄的主要成分姜黄素具有抑瘤作用,可通过逆转IL-6/STAT3介导的EMT来抑制HCC的进展[39]。不仅如此,姜黄素还能通过降低UNC119(lipid-binding chaperone protein uncoordinated 119)的表达抑制Wnt/β-catenin和TGFβ/EMT信号通路,从而抑制肝癌细胞生长和迁移[40]。黄连素是一种生物碱,最近实验[41]发现,黄连素可通过结合TGFβ R受体抑制TGFβ/Smad通路,呈剂量依赖性地抑制肝癌细胞的侵袭和迁移。张彦兵等[42]研究发现,白花蛇舌草总黄酮FOD可逆转TGFβ1诱导MHCC97H肝癌细胞EMT改变,下调细胞侵袭能力。吕美娴等[43]发现裂果薯皂苷单体Ⅰ可以阻断SMMC7721肝癌细胞中TGFβ1/Smad信号通路,抑制EMT发挥拮抗肝癌细胞侵袭转移的作用。刘敏等[44]发现丹参酮ⅡA干预MHCC97H肝癌细胞48 h,使p-Smad3、Snail和N-cadherin、Vimentin降低,Smad7、E-钙黏蛋白和Ep CAM明显升高,抑制肝癌细胞EMT及迁移能力。Qin等[45]发现黄芪甲苷Ⅳ可抑制肝癌细胞Huh7和MHCC97H侵袭迁移,且观察到黄芪甲苷Ⅳ干预的肝癌细胞形态由梭形变为椭圆形,逆转转录因子Slug的上调及EMT相关标记分子的变化,这与其下调细胞Akt和GSK-3β的磷酸化进而抑制β-catenin有关。蛇床子素具有多种药理作用。Lin等[46]发现,蛇床子素可提高E-钙黏蛋白表达,降低N-cadherin、Vimentin水平及MMP2和MMP9的表达,抑制肝癌细胞迁移能力。白藜芦醇是一种非黄酮类多酚有机化合物,近几年研究发现它对多种肿瘤具有抑制作用。研究[47]发现它可以通过上调miR-186-5p,降低Twist1表达,从而抑制肝癌细胞发生EMT,抑制细胞的侵袭迁移。Xing等[48]发现,从铁皮石斛中提取的异紫罗兰素(isoviolanthin)可显著降低TGFβ1处理的肝癌细胞HepG2、BEL-7402的迁移和侵袭能力。进一步研究发现,异紫罗兰素可通过失活TGFβ/Smad和PI3K/Akt/mTOR信号通路,下调Snail、Slug表达,逆转TGFβ1介导的细胞EMT。萝卜硫素是一种富含于十字花科植物的天然化合物,在多种癌症中显示有效抗肿瘤作用。在TGFβ诱导的肝癌细胞中,萝卜硫素干预抑制了细胞侵袭转移及阻止细胞向成纤维细胞形态的改变,逆转细胞的EMT过程[49]。
综上,中药或其来源的化合物均可通过逆转EMT抑制肝癌细胞迁移能力,其调控机制多与TGFβ/Smad或Wnt/β-catenin信号通路有关(表 1、2),但复方或单味药物研究中多缺少具体的信号途径探索。
表 1 中药复方调控EMT抑制HCC转移Table 1. Chinese herbal compound regulates EMT to inhibit HCC metastasis中药复方 药物组成 作用及相关机制 参考文献 鳖甲煎丸 醋鳖甲、射干、鼠妇、大黄、石韦、瞿麦、土鳖虫、蜂房、白芍、桃仁、牡丹皮、浙贝母、厚朴、人参、阿胶、桂枝、干姜、柴胡、黄芩、姜半夏 通过抑制NF-κB活化及snail表达或抑制GSK-3β磷酸化从而降低β-catenin活化水平,抑制EMT [22-23] 健脾化瘀方 人参、白术、山药、茯苓、牡丹皮、丹参、郁金、莪术、柴胡、甘草 下调肝癌细胞Smad蛋白磷酸化水平和Snail表达,抑制EMT [25] 扶正抗癌方 黄芪、灵芝、女贞子、莪术、水蛭、藤梨根、生牡蛎、丹参 抑制MMP,逆转TGFβ1诱导的肝癌细胞EMT [28] 软坚护肝片 山豆根、夏枯草、虎杖、五味子、赤芍、三七等十味中药 抑制TGFβ1诱导的肝癌细胞EMT [30] 松友饮 黄芪、丹参、枸杞、鳖甲、山楂 通过Akt/GSK-3β/β-catenin和TGFβ/Smad通路抑制EMT [32-33] 表 2 单味中药/组分调控EMT抑制HCC转移Table 2. Single Chinese medicine/component regulates EMT to inhibit HCC metastasis组分 来源 作用及相关机制 参考文献 山慈菇含药血清 山慈菇 抑制肝癌细胞EMT转化 [35] 全蝎含药血清/水提物 全蝎 降低Snail表达,逆转肝癌细胞EMT [36] 复方苦参提取液 苦参 抑制Wnt/β-catenin/c-Myc通路,逆转肝癌细胞EMT [37] 银杏叶提取物 银杏叶 下调血小板活化因子,逆转肝癌细胞EMT [38] 姜黄素 姜黄 逆转IL-6/STAT3通路,或减少UNC119蛋白表达而阻断Wnt/β-catenin、TGFβ通路以抑制肝癌细胞EMT [39-40] 黄连素 黄连、黄柏 抑制TGFβ/ Smad通路,抑制肝癌细胞EMT [41] 白花蛇舌草总黄酮 白花蛇舌草 逆转TGFβ1诱导肝癌细胞的EMT [42] 裂果薯皂苷单体Ⅰ 裂果薯 阻断TGFβ/Smad信号通路介导的EMT [43] 丹参酮ⅡA 丹参 调控TGFβ/Smad通路抑制肝癌细胞EMT [44] 黄芪甲苷 黄芪 通过Akt/GSK-3β/β-catenin通路抑制EMT [45] 蛇床子素 蛇床子 抑制EMT,下调MMP2和MMP9表达 [46] 白藜芦醇 黎芦、虎杖、葡萄 上调miR-186-5p,下调Twist1表达,抑制EMT [47] 异紫罗兰素 铁皮石斛 调控TGFβ/Smad和PI3K/Akt/mTOR通路,抑制肝癌细胞发生EMT [48] 萝卜硫素 十字花科(板蓝根、大青叶、葶苈子、莱菔子等) 逆转TGFβ诱导的肝癌细胞EMT [49] 3. 小结与展望
受纤维化、炎性浸润、缺氧等肿瘤微环境影响,包括采用靶向治疗、放疗和手术等标准治疗方法干预导致的微环境变化,多个分子、多种信号通路被激活从而启动或加速肿瘤细胞中EMT转化,在肿瘤转移和侵袭中发挥重要作用,因此调控EMT已成为抑制肿瘤进展的重要途径[50]。目前一些靶向EMT的分子已经或正在进行临床/临床前试验,其药效机制主要包括阻断EMT上游信号通路、应用合成miRNA(如miR-34、miR-200)在转录后水平上干扰EMT-TF。如TGFβRⅠ抑制剂LY-2157299、ALK1小分子抑制剂PF-03446962等正在进行临床试验[50]。但需要注意的是,EMT和间质-上皮转化(MET)之间是动态转换过程,而促进MET可能促进循环肿瘤细胞的定植和转移,导致相反的治疗效果;另外EMT也是参与创伤愈合、组织修复等生理过程中的重要角色,全身性干预可能影响正常生理功能[11]。因此,肿瘤微环境多样性及多种调控通路相互串扰性是更深入了解EMT调控以开发新治疗方法的主要挑战,在针对EMT调控的防治肝癌转移复发药物研究中,需要综合考虑肿瘤细胞异质性、肿瘤微环境进行单个或联合多个信号途径阻断来实现抑制肿瘤进展的效应。
中药或中药联合常规手术和放化疗是治疗肝癌的有效途径[51-52]。研究显示中医药及其来源化合物在防治肝癌转移复发中发挥重要作用,但其相关机制方面研究仍然较少。随着研究人员对EMT在肿瘤进展中的作用和机制的揭示,近年来越来越多的研究发现,一些中药或其组分可调控肿瘤细胞EMT,可能是其发挥抑制肝癌转移复发作用的重要途径。但由于中药的组成成分及药物入血组分的复杂性,对复方或单药进行EMT机制研究多采用含药血清干预肝癌细胞,存在异种血清干扰、实验不稳定性。而单独体内实验不能充分证明中药干预细胞EMT的具体环节,可能对肿瘤微环境、多种信号通路、多个转录因子都具有调控作用,且对信号通路的探讨也有局限性。因此,目前的机制研究多停留在中药对细胞EMT标志物表达水平改变的初步研究上,其中的靶分子或信号通路有待更深入更严谨的实验探讨。开展体内原位肿瘤药物干预实验辅以体外细胞实验能够更有力地证明中药的作用机制,有利于推动中医药单独或联合以更有效地控制包括肝癌在内的肿瘤转移治疗方法的临床应用。此外探讨中药有效成分对EMT的调控机制,也有利于促进防治肝癌转移复发的新型天然药物的研发。
-
表 1 中药复方调控EMT抑制HCC转移
Table 1. Chinese herbal compound regulates EMT to inhibit HCC metastasis
中药复方 药物组成 作用及相关机制 参考文献 鳖甲煎丸 醋鳖甲、射干、鼠妇、大黄、石韦、瞿麦、土鳖虫、蜂房、白芍、桃仁、牡丹皮、浙贝母、厚朴、人参、阿胶、桂枝、干姜、柴胡、黄芩、姜半夏 通过抑制NF-κB活化及snail表达或抑制GSK-3β磷酸化从而降低β-catenin活化水平,抑制EMT [22-23] 健脾化瘀方 人参、白术、山药、茯苓、牡丹皮、丹参、郁金、莪术、柴胡、甘草 下调肝癌细胞Smad蛋白磷酸化水平和Snail表达,抑制EMT [25] 扶正抗癌方 黄芪、灵芝、女贞子、莪术、水蛭、藤梨根、生牡蛎、丹参 抑制MMP,逆转TGFβ1诱导的肝癌细胞EMT [28] 软坚护肝片 山豆根、夏枯草、虎杖、五味子、赤芍、三七等十味中药 抑制TGFβ1诱导的肝癌细胞EMT [30] 松友饮 黄芪、丹参、枸杞、鳖甲、山楂 通过Akt/GSK-3β/β-catenin和TGFβ/Smad通路抑制EMT [32-33] 表 2 单味中药/组分调控EMT抑制HCC转移
Table 2. Single Chinese medicine/component regulates EMT to inhibit HCC metastasis
组分 来源 作用及相关机制 参考文献 山慈菇含药血清 山慈菇 抑制肝癌细胞EMT转化 [35] 全蝎含药血清/水提物 全蝎 降低Snail表达,逆转肝癌细胞EMT [36] 复方苦参提取液 苦参 抑制Wnt/β-catenin/c-Myc通路,逆转肝癌细胞EMT [37] 银杏叶提取物 银杏叶 下调血小板活化因子,逆转肝癌细胞EMT [38] 姜黄素 姜黄 逆转IL-6/STAT3通路,或减少UNC119蛋白表达而阻断Wnt/β-catenin、TGFβ通路以抑制肝癌细胞EMT [39-40] 黄连素 黄连、黄柏 抑制TGFβ/ Smad通路,抑制肝癌细胞EMT [41] 白花蛇舌草总黄酮 白花蛇舌草 逆转TGFβ1诱导肝癌细胞的EMT [42] 裂果薯皂苷单体Ⅰ 裂果薯 阻断TGFβ/Smad信号通路介导的EMT [43] 丹参酮ⅡA 丹参 调控TGFβ/Smad通路抑制肝癌细胞EMT [44] 黄芪甲苷 黄芪 通过Akt/GSK-3β/β-catenin通路抑制EMT [45] 蛇床子素 蛇床子 抑制EMT,下调MMP2和MMP9表达 [46] 白藜芦醇 黎芦、虎杖、葡萄 上调miR-186-5p,下调Twist1表达,抑制EMT [47] 异紫罗兰素 铁皮石斛 调控TGFβ/Smad和PI3K/Akt/mTOR通路,抑制肝癌细胞发生EMT [48] 萝卜硫素 十字花科(板蓝根、大青叶、葶苈子、莱菔子等) 逆转TGFβ诱导的肝癌细胞EMT [49] -
[1] SUNG H, FERLAY J, SIEGEL RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA Cancer J Clin, 2021, 71(3): 209-249. DOI: 10.3322/caac.21660. [2] ZHAO P, JIANG DM, XIAN LF, et al. Mortality analysis of primary liver cancer in the mainland of China from 2004 to 2018[J]. Shanghai J Prev Med, 2021, 33: 881-886. DOI: 10.19428/j.cnki.sjpm.2021.21080.赵沛, 蒋栋铭, 鲜林峰, 等. 2004—2018年中国大陆地区原发性肝癌死亡率分析[J]. 上海预防医学, 2021, 33: 881-886. DOI: 10.19428/j.cnki.sjpm.2021.21080. [3] UCHINO K, TATEISHI R, SHⅡNA S, et al. Hepatocellular carcinoma with extrahepatic metastasis: clinical features and prognostic factors[J]. Cancer, 2011, 117(19): 4475-4483. DOI: 10.1002/cncr.25960. [4] PINZANI M. Epithelial-mesenchymal transition in chronic liver disease: fibrogenesis or escape from death?[J]. J Hepatol, 2011, 55(2): 459-465. DOI: 10.1016/j.jhep.2011.02.001. [5] MITTAL V. Epithelial mesenchymal transition in tumor metastasis[J]. Annu Rev Pathol, 2018, 13: 395-412. DOI: 10.1146/annurev-pathol-020117-043854. [6] LAMOUILLE S, XU J, DERYNCK R. Molecular mechanisms of epithelial-mesenchymal transition[J]. Nat Rev Mol Cell Biol, 2014, 15(3): 178-196. DOI: 10.1038/nrm3758. [7] CHEN J, CAO SW, CAI Z, et al. Epithelial-mesenchymal transition phenotypes of circulating tumor cells correlate with the clinical stages and cancer metastasis in hepatocellular carcinoma patients[J]. Cancer Biomark, 2017, 20(4): 487-498. DOI: 10.3233/CBM-170315. [8] HELLER M, PARIKH ND, FIDELMAN N, et al. Frontiers of therapy for hepatocellular carcinoma[J]. Abdom Radiol (NY), 2021, 46(8): 3648-3659. DOI: 10.1007/s00261-021-03065-0. [9] LIAO X, BU Y, JIA Q. Traditional Chinese medicine as supportive care for the management of liver cancer: Past, present, and future[J]. Genes Dis, 2020, 7(3): 370-379. DOI: 10.1016/j.gendis.2019.10.016. [10] LI XH, YUAN HX. Improving the efficacy of traditional Chinese medicine in treatment of primary liver cancer based on etiology and pathogenesis[J]. J Clin Hepatol, 2021, 37(9): 2001-2004. DOI: 10.3969/j.issn.1001-5256.2021.09.001.李秀惠, 袁慧鑫. 从病因病机入手提高中医药治疗原发性肝癌的疗效[J]. 临床肝胆病杂志, 2021, 37(9): 2001-2004. DOI: 10.3969/j.issn.1001-5256.2021.09.001. [11] BRABLETZ S, SCHUHWERK H, BRABLETZ T, et al. Dynamic EMT: a multi-tool for tumor progression[J]. EMBO J, 2021, 40(18): e108647. DOI: 10.15252/embj.2021108647. [12] KANG E, SEO J, YOON H, et al. The post-translational regulation of epithelial-mesenchymal transition-inducing transcription factors in cancer metastasis[J]. Int J Mol Sci, 2021, 22(7): 3591. DOI: 10.3390/ijms22073591. [13] LIU Z, WANG Y, DOU C, et al. Hypoxia-induced up-regulation of VASP promotes invasiveness and metastasis of hepatocellular carcinoma[J]. Theranostics, 2018, 8(17): 4649-4663. DOI: 10.7150/thno.26789. [14] DONG Y, ZHENG Q, WANG Z, et al. Higher matrix stiffness as an independent initiator triggers epithelial-mesenchymal transition and facilitates HCC metastasis[J]. J Hematol Oncol, 2019, 12(1): 112. DOI: 10.1186/s13045-019-0795-5. [15] GEORGAKOPOULOS-SOARES I, CHARTOUMPEKIS DV, KYRIAZOPOULOU V, et al. EMT factors and metabolic pathways in cancer[J]. Front Oncol, 2020, 10: 499. DOI: 10.3389/fonc.2020.00499. [16] HAO Y, BAKER D, TEN DIJKE P. TGF-β-mediated epithelial-mesenchymal transition and cancer metastasis[J]. Int J Mol Sci, 2019, 20(11) : 2767. DOI: 10.3390/ijms20112767. [17] DERYNCK R, BUDI EH. Specificity, versatility, and control of TGF-β family signaling[J]. Sci Signal, 2019, 12(570): eaav5183. DOI: 10.1126/scisignal.aav5183. [18] GURZU S, KOBORI L, FODOR D, et al. Epithelial mesenchymal and endothelial mesenchymal transitions in hepatocellular carcinoma: a review[J]. Biomed Res Int, 2019, 2019: 2962580. DOI: 10.1155/2019/2962580. [19] DONGRE A, WEINBERG RA. New insights into the mechanisms of epithelial-mesenchymal transition and implications for cancer[J]. Nat Rev Mol Cell Biol, 2019, 20(2): 69-84. DOI: 10.1038/s41580-018-0080-4. [20] HAN TS, HUR K, CHO HS, et al. Epigenetic associations between lncRNA/circRNA and miRNA in hepatocellular carcinoma[J]. Cancers (Basel), 2020, 12(9): 2622. DOI: 10.3390/cancers12092622. [21] TSUBAKIHARA Y, MOUSTAKAS A. Epithelial-mesenchymal transition and metastasis under the control of transforming growth factor β[J]. Int J Mol Sci, 2018, 19(11): 3672. DOI: 10.3390/ijms19113672. [22] PENG T. Clinical application of Biejiajian Pill in the treatment of liver cancer[J]. Chin J Integr Tradit West Med Liver Dis, 2020, 30(6): 481-483. DOI: 10.3969/j.issn.1005-0264.2020.06.001.彭涛. 鳖甲煎丸在肝癌治疗中的临床应用[J]. 中西医结合肝病杂志, 2020, 30(6): 481-483. DOI: 10.3969/j.issn.1005-0264.2020.06.001. [23] ZHONG XD, WEN B, SUN HT, et al. Mechanism of Biejiajian Wan against EMT of hepatocellular carcinoma cells through NF-κB signaling pathway[J]. Chin J Exp Med Formul, 2022, 28(1): 24-32. DOI: 10.13422/j.cnki.syfjx.20212421.钟晓丹, 文彬, 孙海涛, 等. 鳖甲煎丸通过NF-κB信号通路抑制肝癌细胞上皮间质转化的作用机制[J]. 中国实验方剂学杂志, 2022, 28(1): 24-32. DOI: 10.13422/j.cnki.syfjx.20212421. [24] ZHAO WT. Mechanism of Wnt/β-catenin signaling pathway regulating WB-F344 cell epithelial mesenchymal transition and intervention of Biejiajian pills[D]. Guangzhou: Southern Medical University, 2021.招文婷. Wnt/β-catenin信号通路调控WB-F344细胞上皮间质转化的分子机制及鳖甲煎丸的干预作用[D]. 广州: 南方医科大学, 2021. [25] ZHONG C, HUANG JH, CHEN QY, et al. Effects of Jianpi Huayu formula on Smad7 protein and epithelial mesenchymal transition of hepatocellular carcinoma cell[J]. China J Tradit Chin Med Pharm, 2017, 32(8): 3789-3792. https://www.cnki.com.cn/Article/CJFDTOTAL-BXYY201708124.htm钟崇, 黄俊海, 陈秋源, 等. 健脾化瘀法方药对肝癌细胞Smad7蛋白及上皮间质转化的影响[J]. 中华中医药杂志, 2017, 32(8): 3789-3792. https://www.cnki.com.cn/Article/CJFDTOTAL-BXYY201708124.htm [26] FENG KL, CHEN QL, XIE CF, et al. JianpiHuayu decoction inhibits migration, invasion and EMT of hepatomacarcinoma cell through regulation of exosome[J]. Tradit Chin Drug Res Clin Pharmacol, 2021, 32(12): 1745-1751. DOI: 10.19378/j.issn.1003-9783.2021.12.002.冯坤良, 陈清莲, 谢春凤, 等. 健脾化瘀方体外通过外泌体影响肝癌细胞的迁移、侵袭及上皮间质转化[J]. 中药新药与临床药理, 2021, 32(12): 1745-1751. DOI: 10.19378/j.issn.1003-9783.2021.12.002. [27] CHEN K, ZHANG YF. Clinical curative observation of using Jianpi Huayu decoction combined with chemotherapy in the treatment of 78cases of primary hepatocellular carcinoma (liver-depression and spleen-deficiency syndrome)[J]. J Sichuan Tradit Chin Med, 2017, 35(7): 108-110. https://www.cnki.com.cn/Article/CJFDTOTAL-SCZY201707046.htm陈科, 张月峰. 健脾化瘀方联合化疗治疗原发性肝癌(肝郁脾虚型)78例临床疗效观察[J]. 四川中医, 2017, 35(7): 108-110. https://www.cnki.com.cn/Article/CJFDTOTAL-SCZY201707046.htm [28] FANG Y, XIAO HJ, LI RT, et al. Effect of Fuzheng Kang'ai formula on anti-hepatoma by reversing EMT transformation of HepG2 cells[J]. Guid J Tradit Chin Med Pharm, 2019, 25(1): 60-63. https://www.cnki.com.cn/Article/CJFDTOTAL-HNZB201901015.htm方瑜, 肖海娟, 李仁廷, 等. 扶正抗癌方通过逆转HepG2细胞EMT抗肝癌的实验研究[J]. 中医药导报, 2019, 25(1): 60-63. https://www.cnki.com.cn/Article/CJFDTOTAL-HNZB201901015.htm [29] LIANG XJ, LIANG ST, JIANG WH, et al. Overview of the pharmacological effects of Ruanjian Hugan table and their experimental and clinical application in the treatment of liver diseases[J]. Intern Med China, 2020, 15(5): 568-570. DOI: 10.16121/j.cnki.cn45-1347/r.2020.05.17.梁雪婧, 梁水庭, 蒋汶洪, 等. 软坚护肝片的药理学作用及其在肝脏疾病治疗中的实验和临床应用研究概述[J]. 内科, 2020, 15(5): 568-570. DOI: 10.16121/j.cnki.cn45-1347/r.2020.05.17. [30] SUN Z, LI S, CHENG BB, et al. Research of mechanisms of Ruanjian Hugan table on inhibition of hepatocellular carcinoma migration and the epithelial mesenchymal cell transformation[J]. J Liaoning Univ Tradit Chin Med, 2020, 22(3): 22-25. DOI: 10.13194/j.issn.1673-842x.2020.03.007.孙振, 李书, 程彬彬, 等. 软坚护肝片含药血清对肝癌细胞迁移侵袭作用及机制[J]. 辽宁中医药大学学报, 2020, 22(3): 22-25. DOI: 10.13194/j.issn.1673-842x.2020.03.007. [31] ZHANG QB, MENG XT, JIA QA, et al. Herbal compound Songyou Yin and moderate swimming suppress growth and metastasis of liver cancer by enhancing immune function[J]. Integr Cancer Ther, 2016, 15(3): 368-375. DOI: 10.1177/1534735415622011. [32] ZHANG N, WANG LR, LI DD, et al. Interferon-α combined with herbal compound "Songyou Yin" effectively inhibits the increased invasiveness and metastasis by insufficient radiofrequency ablation of hepatocellular carcinoma in an animal model[J]. Integr Cancer Ther, 2018, 17(4): 1260-1269. DOI: 10.1177/1534735418801525. [33] ZHENG S, JIA Q, SHEN H, et al. Treatment with the herbal formula Songyou Yin inhibits epithelial-mesenchymal transition in hepatocellular carcinoma through downregulation of TGF-β1 expression and inhibition of the SMAD2/3 signaling pathway[J]. Oncol Lett, 2017, 13(4): 2309-2315. DOI: 10.3892/ol.2017.5700. [34] QIU JX, YANG JK. Use of "SRRS" recipe on treatment of late stage liver cancer patients and their experimental investigations[J]. Chin J Integr Tradit West Med, 1987, 7(5): 275-277. https://www.cnki.com.cn/Article/CJFDTOTAL-ZZXJ198705008.htm邱佳信, 杨金坤. 健脾理气、清热解毒、软坚化痰方剂治疗晚期肝癌的临床观察及实验研究[J]. 中西医结合杂志, 1987, 7(5): 275-277. https://www.cnki.com.cn/Article/CJFDTOTAL-ZZXJ198705008.htm [35] CHENG QB, YANG YP, WANG Y, et al. Effect of pseudobulbus cremastrae seu pleiones on apoptosis and epithelial-mesenchymal transition of hepatoma carcinoma cell[J]. Chin Med, 2021, 36(10): 2202-2207. DOI: 10.16368/j.issn.1674-8999.2021.10.459.程清波, 杨艳萍, 王滢, 等. 山慈菇对肝癌细胞凋亡和上皮间质转化的影响[J]. 中医学报, 2021, 36(10): 2202-2207. DOI: 10.16368/j.issn.1674-8999.2021.10.459. [36] YAN YQ, XIE J, WANG JF, et al. Scorpion inhibits epithelial-mesenchymal transition and metastasis of hepatocellular carcinoma[J]. Exp Biol Med (Maywood), 2018, 243(7): 645-654. DOI: 10.1177/1535370218762514. [37] WANG KX, DU GH, QIN XM, et al. Compound Kushen Injection intervenes metabolic reprogramming and epithelial-mesenchymal transition of HCC via regulating β-catenin/c-Myc signaling[J]. Phytomedicine, 2021, 93: 153781. DOI: 10.1016/j.phymed.2021.153781. [38] FU J, LIU JL, LIU KJ, et al. Ginkgo biloba extract contains a mechanism by which medicated serum indirectly inhibits malignant progression of hepatocellular carcinoma cell lines[J]. J Ningxia Med Univ, 2021, 43(7): 665-770. DOI: 10.16050/j.cnki.issn1674-6309.2021.07.001.付江, 刘敬莉, 柳科军, 等. 银杏叶提取物含药血清间接抑制肝癌细胞株恶性进展的机制[J]. 宁夏医科大学学报, 2021, 43(7): 665-770. DOI: 10.16050/j.cnki.issn1674-6309.2021.07.001. [39] CAO W, ZHANG Y, LI A, et al. Curcumin reverses hepatic epithelial mesenchymal transition induced by trichloroethylene by inhibiting IL-6R/STAT3[J]. Toxicol Mech Methods, 2021, 31(8): 589-599. DOI: 10.1080/15376516.2021.1941463. [40] ZHAO Z, MALHOTRA A, SENG WY. Curcumin modulates hepatocellular carcinoma by reducing UNC119 expression[J]. J Environ Pathol Toxicol Oncol, 2019, 38(3): 195-203. DOI: 10.1615/JEnvironPatholToxicolOncol.2019029549. [41] DU H, GU J, PENG Q, et al. Berberine suppresses EMT in liver and gastric carcinoma cells through combination with TGFβR regulating TGF-β/Smad pathway[J]. Oxid Med Cell Longev, 2021, 2021: 2337818. DOI: 10.1155/2021/2337818. [42] ZHANG YB, ZHU J, XIAO JX, et al. Effects of ethanol extracts of hedyotis diffusa on proliferation of rat hepatocarcinoma cells by regulating DDR1[J]. J Xi'an Jiaotong Univ: Med Sci, 2016, 37(2): 279-282. DOI: 10.7652/jdyxb201602027.张彦兵, 朱娇, 肖菊香, 等. 白花蛇舌草总黄酮对TGF-β1诱导的肝癌MHCC97-H细胞EMT的逆转作用及其机制[J]. 西安交通大学学报(医学版), 2016, 37(2): 279-282. DOI: 10.7652/jdyxb201602027. [43] LYU MX, LIAO ZH, ZHOU H, et al. Saponin monomer I of Schizocapsa plantaginea Hance effect of epithelial mesenchymal transformation on the invasion and metastasis of human hepatocellular carcinoma SMMC-7721 by TGF-β1[J]. Chin Pharmacol Bull, 2020, 36(3): 408-413. DOI: 10.3969/j.issn.1001-1978.2020.03.021.吕美娴, 廖智红, 周欢, 等. 裂果薯皂苷单体Ⅰ通过TGF-β1调控上皮间质转化对人肝癌细胞SMMC-7721侵袭和转移的影响[J]. 中国药理学通报, 2020, 36(3): 408-413. DOI: 10.3969/j.issn.1001-1978.2020.03.021. [44] LIU M, XIONG CM, WANG ZY, et al. Effect of tanshinone ⅡA on phosphorylation of Smad3/Smad7 protein and epithelial mesenchymal transition of hepatocellular carcinoma cell line MHCC97H[J]. Guid J Tradit Chin Med Pharm, 2017, 23(10): 30-34. DOI: 10.13862/j.cnki.cn43-1446/r.2017.10.011.刘敏, 熊成名, 王子豫, 等. 丹参酮ⅡA对肝癌细胞MHCC97H Smad3/Smad7蛋白及上皮间质转化的影响[J]. 中医药导报, 2017, 23(10): 30-34. DOI: 10.13862/j.cnki.cn43-1446/r.2017.10.011. [45] QIN CD, MA DN, REN ZG, et al. Astragaloside IV inhibits metastasis in hepatoma cells through the suppression of epithelial-mesenchymal transition via the Akt/GSK-3β/β-catenin pathway[J]. Oncol Rep, 2017, 37(3): 1725-1735. DOI: 10.3892/or.2017.5389. [46] LIN ZK, LIU J, JIANG GQ, et al. Osthole inhibits the tumorigenesis of hepatocellular carcinoma cells[J]. Oncol Rep, 2017, 37(3): 1611-1618. DOI: 10.3892/or.2017.5403. [47] SONG FF, ZHANG YW, PAN ZF, et al. Resveratrol inhibits the migration, invasion and epithelialmesenchymal transition in liver cancer cells through upregulating miR-186-5p expression in vitro[J]. J Zhejiang Univ Med Sci, 2021, 50(5): 582-590. DOI: 10.3724/zdxbyxb-2021-0197.宋飞凤, 张轶雯, 潘宗富, 等. 白藜芦醇通过上调miR-186-5p表达抑制肝癌细胞迁移、侵袭和上皮-间质转化[J]. 浙江大学学报, 2021, 50(5): 582-590. DOI: 10.3724/zdxbyxb-2021-0197. [48] XING S, YU W, ZHANG X, et al. Isoviolanthin extracted from dendrobium officinale reverses TGF-β1-mediated epithelial-mesenchymal transition in hepatocellular carcinoma cells via deactivating the TGF-β/Smad and PI3K/Akt/mTOR signaling pathways[J]. Int J Mol Sci, 2018, 19(6): 1556. DOI: 10.3390/ijms19061556. [49] LIU P, ATKINSON SJ, AKBAREIAN SE, et al. Sulforaphane exerts anti-angiogenesis effects against hepatocellular carcinoma through inhibition of STAT3/HIF-1α/VEGF signalling[J]. Sci Rep, 2017, 7(1): 12651. DOI: 10.1038/s41598-017-12855-w. [50] CHO ES, KANG HE, KIM NH, et al. Therapeutic implications of cancer epithelial-mesenchymal transition (EMT)[J]. Arch Pharm Res, 2019, 42(1): 14-24. DOI: 10.1007/s12272-018-01108-7. [51] GAO YR, CHEN SJ, HOU YW, et al. Clinical effect of Huaier Granule sequential with radiofrequency ablation and TACE in treating primary hepatic carcinoma[J]. J Changchun Univ Chin Med, 2020, 36(4): 684-687. DOI: 10.13463/j.cnki.cczyy.2020.04.021.高远韧, 陈思佳, 侯英文, 等. TACE联合射频消融术序贯槐耳颗粒治疗原发性肝癌[J]. 长春中医药大学学报, 2020, 36(4): 684-687. DOI: 10.13463/j.cnki.cczyy.2020.04.021. [52] WANG H, SHAN L, WU XX, et al. Clinical efficacy of Qinggan Quai prescription combined with transcatheter arterial chemoembolization in treatment of advanced liver cancer with heat stasis[J]. Clin J Med Offic, 2021, 49(3): 306-308. DOI: 10.16680/j.1671-3826.2021.03.23.王昊, 单良, 吴孝雄, 等. 清肝祛癌方联合肝动脉化疗栓塞术治疗晚期热瘀型肝癌临床疗效[J]. 临床军医杂志, 2021, 49(3): 306-308. DOI: 10.16680/j.1671-3826.2021.03.23. 期刊类型引用(3)
1. 谭钦文,黄晶晶,钟瑞熙,杜沅沁,徐健,农金丽,彭玉姣. 鳖甲煎丸调控AKT/mTOR信号通路在肝癌细胞有氧糖酵解中的作用. 临床肝胆病杂志. 2025(02): 300-306 .  本站查看
本站查看2. 孙嘉玲,冯文杏,张仁杰,孙新锋,彭蓝芬,刘兴宁,张卫,蔡本强,周小舟. 芪术抗癌方通过STAT3信号通路抑制肝癌细胞Huh-7上皮间质转化的作用机制. 中华中医药杂志. 2025(02): 612-618 .  百度学术
百度学术3. 罗燕,唐蔚,陈玲珑,曾千,张轩,罗吉. 肝喜片通过miR200a/ZEB信号通路抑制肝癌细胞HepG2上皮间质转化的机制研究. 湖南中医药大学学报. 2024(11): 2007-2013 .  百度学术
百度学术其他类型引用(2)
-




 PDF下载 ( 2270 KB)
PDF下载 ( 2270 KB)


 下载:
下载:

 下载:
下载:
 百度学术
百度学术