P-I-R分型和Laennec分级与乙型肝炎肝硬化患者抗病毒治疗后组织学和预后的关系
DOI: 10.3969/j.issn.1001-5256.2023.03.015
Association of P-I-R classification and Laennec grading with histology and prognosis after antiviral therapy in patients with hepatitis B cirrhosis
-
摘要:
目的 研究P-I-R分型和Laennec分级评价乙型肝炎肝硬化患者接受抗病毒治疗后的组织学改变,及两种评价系统与临床预后的关系。 方法 连续筛选2013年10月—2014年10月来自14个中心的218例患者,病理(Ishak评分≥5分)诊断肝硬化接受抗病毒治疗72周完成2次肝组织活检,并符合P-I-R分型标准。218例患者分为无肝细胞癌(HCC)组(n=186)和HCC组(n=32)。计数资料组间比较采用χ2检验和Fisher精确检验。比较抗病毒治疗后HCC发生情况时,连续变量采用非参数检验Mann-Whitney U检验;比较P-I-R分型与Laennec分级不同组间差异时,连续变量采用非参数检验Kruskal-Wallis H检验。采用单因素和多因素Cox比例风险回归分析并计算风险比(HR)和95%CI。采用Kaplan-Meier法计算HCC的累积发生率。 结果 抗病毒治疗72周后无HCC组和HCC组间P-I-R分型情况比较差异有统计学意义(P<0.001)。抗病毒治疗前后Laennec分级和P-I-R分型的分布均有统计学差异(P值均<0.001)。抗病毒治疗后,按照Laennec分级分为4A组(n=33)、4B组(n=71)、4C组(n=114),3组间PLT(H=36.429,P<0.001)、LSM(H=13.983, P=0.004)、Ishak评分(χ2=23.060, P<0.001)、HAI评分(P<0.001)比较差异均有统计学意义。抗病毒治疗72周后,按照P-I-R分型分为R组(n=70)、I组(n=52)和P组(n=96),3组间PLT(H=7.193,P=0.028)、LSM(H=6.238, P=0.045)、Ishak评分(χ2=7.986, P<0.001)、HAI评分(P=0.002)、HCC发生情况(P<0.001)比较,差异均有统计学意义。P-I-R分型P组和R组HCC发生率有显著差异(HR=24.21; 95%CI: 0.46~177.99, P=0.002)。经过调整其他混杂因素后,P-I-R分型是预测HCC发生的独立指标(HR=12.69; 95%CI: 4.63~34.80, P=0.002)。 结论 P-I-R分型和Laennec分级均能反应患者抗病毒治疗前后纤维化的特征及改变情况,其中P-I-R分型对抗病毒治疗后纤维化的改变更敏感。P-I-R分型(治疗后)可用于预测抗病毒治疗后患者HCC发生的风险。 Abstract:Objective To investigate the role of P-I-R classification and Laennec grading in evaluating histological changes in patients with hepatitis B cirrhosis after receiving antiviral therapy, as well as the association of these two evaluation systems with clinical prognosis. Methods A total of 218 patients from 14 centers were consecutively screened from October 2013 to October 2014, and these patients were diagnosed with liver cirrhosis based on pathology (Ishak score ≥5), received antiviral therapy for 72 weeks, completed two liver biopsies, and met the P-I-R classification criteria. The 218 patients were divided into non-hepatocellular carcinoma (HCC) group with 186 patients and HCC group with 32 patients. The chi-square test and the Fisher's exact test were used for comparison of categorical data between groups. For the comparison of HCC after antiviral therapy, the non-parametric Mann-Whitney U test was used for continuous variables, and for the comparison of P-I-R classification and Laennec grading, the non-parametric Kruskal-Wallis H test was used for continuous variables. Univariate and multivariate Cox regression analyses were used to calculate hazard ratio (HR) and 95% confidence interval (CI), and the Kaplan-Meier method was used to calculate the cumulative incidence rate of HCC. Results After 72 weeks of antiviral therapy, there was a significant difference in P-I-R classification between the non-HCC group and the HCC group (P < 0.001). There were significant differences in the distribution of Laennec grading and P-I-R classification before and after antiviral therapy (P < 0.001). After antiviral therapy, the 218 patients were divided into 4A group with 33 patients, 4B group with 71 patients, and 4C group with 114 patients according to Laennec grading, and there were significant differences between these three groups in platelet count (PLT) (H=36.429, P < 0.001), liver stiffness measurement (LSM) (H=13.983, P=0.004), Ishak score (χ2=23.060, P < 0.001), and HAI score (P < 0.001). After antiviral therapy, the 218 patients were divided into R group with 70 patients, I group with 52 patients, and P group with 96 patients according to P-I-R classification, and there were significant differences between these three groups in PLT (H=7.193, P=0.028), LSM (H=6.238, P=0.045), Ishak score (χ2=7.986, P < 0.001), HAI score (P=0.002), and HCC (P < 0.001). There was a significant difference in the incidence rate of HCC between the P and R groups based on P-I-R classification (HR=24.21, 95%CI: 0.46-177.99, P=0.002). After adjustment for other confounding factors, P-I-R classification was an independent predictive factor for HCC (HR=12.69, 95%CI: 4.63-34.80, P=0.002). Conclusion Both P-I-R classification and Laennec grading can reflect the features and changes of fibrosis before and after antiviral therapy, and P-I-R classification is more sensitive to fibrosis changes after antiviral therapy. P-I-R classification (after treatment) can be used to assess the risk of HCC in patients after antiviral therapy. -
Key words:
- Hepatitis B /
- Liver Cirrhosis /
- Carcinoma, Hepatocellular /
- P-I-R Typing /
- Laennec Classification
-
肝硬化是慢性肝病监测管理的关键阶段,其病因包括嗜肝病毒感染、饮酒、脂肪浸润等[1]。在慢性病毒性肝炎抗病毒治疗下,研究证实肝硬化组织病理学不是同质和静态的,而是有时空异质性和动态变化的[2-5]。也有研究[6]证实肝硬化病理分级可预测肝细胞癌(HCC)的发生,这成为肝硬化患者监测和干预的热点。尽管肝活检的有创性影响其广泛应用,但仍是肝脏炎症和纤维化评估的金标准[7]。肝纤维化的病理评分系统包括Knodell组织活动指数(HAI)、Bats-ludwig分期、Scheuer、METAVIR和Ishak系统等[8-11],但以上评分系统均缺少对肝硬化阶段的进一步分层。2000年开发的Laennec组织病理学分级(Laennec分级)弥补了这一空白,它是基于METAVIR系统,将肝硬化阶段(METAVIR系统4级)根据纤维间隔的厚度和结节的大小分为4A、4B、4C,实现了对肝硬化阶段进行分层。已有研究[12-14]发现Laennec分级可预测肝硬化患者发生肝脏相关事件(失代偿期肝硬化等)的风险,但尚无研究证实其具有评估肝纤维化动态改变能力,对于其与乙型肝炎肝硬化患者抗病毒治疗后HCC发生率的相关性尚无深入研究。
P-I-R分型(P-I-R北京分型)是2017年由贾继东教授首先提出, 通过分析慢性乙型肝炎(CHB)患者抗病毒治疗前后的肝纤维化特点建立的一种可以判定肝纤维动态变化的评分系统[15]。该系统将Ishak≥3患者肝活检分为肝纤维进展型(progressive,P)、不确定型(intermediate,I)和逆转型(regressive,R)。小样本数据显示,与R组相比,P或I组患者发生肝纤维持续进展的风险增加[15]。但其对乙型肝炎肝硬化患者抗病毒治疗后肝纤维化的动态变化和肝脏相关事件包括HCC的预测能力缺少相关研究。此外,P-I-R分型与Laennec分级在乙型肝炎肝硬化病理相关性方面的比较缺少研究数据。
本研究通过一项前瞻性多中心随机对照临床研究数据分析P-I-R分型和Laennec分级对乙型肝炎肝硬化患者抗病毒治疗后肝纤维化动态变化的反应能力,并进一步预测HCC发生率。
1. 资料和方法
1.1 研究对象
本研究数据来源于一项长期随访的前瞻性多中心随机对照临床试验(CHBALT-F,注册号:NCT01965418)。CHBALT-F试验的临床设计和主要结果已在相关文献[16-17]中详细描述。该试验在2013年10月—2014年10月,连续筛选来自14个中心的患者,进入随机队列的患者以1∶1的比例被随机分配到对照组(恩替卡韦0.5 mg/d,安慰剂2.0 g/次, 每天3次)和治疗组(恩替卡韦0.5 mg/d合并鳖甲软肝片2.0 g/次, 每天3次)。纳入标准:(1)年龄在18~65岁,符合乙型肝炎诊断标准(至少6个月HBsAg阳性);(2)治疗前和治疗后进行2次肝活检,并前后2次活检Ishak评分≥5的肝硬化患者;(3)符合肝硬化抗病毒标准,HBV DNA可检测;(4)未曾接受过抗HBV药物治疗或过去1年内未接受核苷酸类似物治疗,对恩替卡韦无耐药性;(5)近6个月内未接受过抗肝纤维化药物治疗。排除标准:(1)无完整随访资料;(2)标本染色和质量缺陷不能进行P-I-R评分和Laennec评分;(3)合并其他病毒性肝炎、慢性重度肝炎、失代偿性肝硬化、肝癌等严重或终末期肝病;心脏、肾、肺、内分泌、血液、代谢、胃肠道严重原发疾病或精神患者;(4)孕妇和哺乳期患者;(5)恩替卡韦或鳖甲软肝片药物过敏或超敏反应。
1.2 临床和实验室指标
在双盲期,每3个月进行1次临床、实验室检查和不良事件评估,之后每6个月进行1次。肝活检样本在基线(筛查前3个月内)和双盲试验的第72周采集。血清HBV DNA水平采用COBAS TaqMan法(Roche,美国)测定,定量下限为20 IU/mL。基线HBsAg水平采用化学发光免疫分析法(Abbott,USA)测定。肝硬度测量值(LSM)使用纤维扫描(Echosens,法国)进行。LSM减少定义为治疗后减去治疗前小于-30%;LSM稳定定义为:-30%≤治疗后减去治疗前≤30%;LSM增加定义为治疗后减去治疗前>30%。
1.3 肝活检和组织学评估
肝活检在超声引导下进行,并遵循标准方案,采用16G快速切割或Menghini针(McGaw Park,美国)。为了能正确评估Ishak纤维化评分、P-I-R分型及Laennec分级,控制活检样本质量:收集至少两条长度不小于20 mm的肝组织以确保至少有11个门静脉束。所有标本均用福尔马林和石蜡固定。在一块4 mm厚的组织上进行HE染色、马松三色染色和银染。所有肝活检样本由2名肝脏病理学家在独立的病理中心盲审。根据核心长度和门静脉束的数量判断活检样本质量的有效性。组织学评估包括4个主要部分:(1)Ishak纤维化评分评估纤维化阶段(≥5表示肝硬化);(2)Ishak改良的HAI分级系统评估炎症活动(≤3表示轻度或无坏死炎症,≥13表示严重坏死炎症);(3) 根据之前发表的标准[8],Ishak纤维化评分为5和6(肝硬化)的患者根据Laennec分级系统进一步分为4A、4B和4C(4A,轻度肝硬化,明确或可能;4B,中度肝硬化,至少2个宽间隔;4C,严重肝硬化,至少一个非常宽的间隔或许多微小结节)。“宽隔”和“非常宽的隔”是根据厚度的相对比较定义的,“宽隔”定义为窄于结节,而“非常宽的隔”定义为宽于结节;(4)P-I-R纤维化评分分为进展为主型(P组)、不确定型(I组)、逆转为主型(R组),其中P组定义为50%以上的肝活检标本为广泛而松散的胶原纤维,三色染色显示浅色和致密的深蓝色染色纤维混合,R组定义为50%以上的肝活检标本中胶原纤维纤细而致密,在三色染色上主要表现为致密的深蓝色,I组定义为50%的广泛而松散的胶原纤维和50%的纤细而致密的胶原纤维。
1.4 随访
HCC监测方式为每6个月肝脏超声检查和甲胎蛋白血液检查1次。HCC诊断采用多相动态对比增强磁共振成像或计算机断层扫描,并经肝活检证实,分期依据为巴塞罗那临床肝癌(BCLC)分期系统。研究记录末次随访时间为2021年5月。
1.5 统计学方法
采用R 4.1.2和SPSS 26.0统计软件进行数据分析。符合正态分布的计量资料采用x±s表示,非正态分布的计量资料采用M(最小值~最大值)表示;计数资料组间比较采用χ2检验和Fisher精确检验。比较抗病毒治疗后HCC组和无HCC组间差异时,连续变量采用非参数检验Mann-Whitney U检验;比较P-I-R分型与Laennec分级不同组间差异时,连续变量采用非参数检验Kruskal-Wallis H检验。采用单因素和多因素Cox比例风险回归分析并计算风险比(HR)和95%CI。采用Kaplan-Meier法计算HCC的累积发生率。P<0.05为差异有统计学意义。
2. 结果
2.1 一般资料
共收集患者218例(图 1),分为无HCC组(n=186)和HCC组(n=32)。基线时,2组间PLT和HBV DNA水平比较差异均有统计学意义(P值均<0.05)(表 1)。
表 1 无HCC组和HCC组基线资料的比较Table 1. Comparison of baseline data between the non-HCC group and the HCC group项目 总体(n=218) 无HCC组(n=186) HCC组(n=32) 统计值 P值 治疗方式[例(%)] χ2=0.476 0.097 ETV+PLC 117(53.7) 95 (51.1) 22(68.8) ETV+BJRG 101(46.3) 91(48.9) 10 (31.3) 年龄(岁) 46.0(19.0~68.0) 46.0(19.0~68.0) 47.0(34.0~63.0) U=2833 0.664 男性[例(%)] 157(72.0) 133(71.5) 24(75.0) χ2=0.837 0.846 饮酒史[例(%)] 39(17.9) 30(16.1) 9(28.1) χ2=2.207 0.166 BMI(kg/m2) 23.6(15.8~36.3) 23.7(15.8~36.3) 23.0(17.7~32.7) U=3070 0.777 Alb(g/L) 41.0(26.9~50.0) 41.0 (26.9~50.0) 42.0(30.4~48.0) U=2935 0.903 TBil(μmol/L) 15.2(5.0~149.0) 14.4(5.0~149.0) 17.4(6.1~47.9) U=2348 0.057 ALT(U/L) 50.5(10.0~1370.0) 49.0(10.0~1370.0) 66.5(13.0~854.0) U=2473 0.128 AST(U/L) 46.0(17.0~836.0) 45.0(17.0~836.0) 55.0(20.0~635.0) U=2415 0.089 PLT(×109/L) 125.0(45.0~277.0) 130.0(45.0~277.0) 116.0(50.0~223.0) U=3652 0.040 AFP(ng/mL) 7.00(0.91~200.00) 7.00(0.91~200.00) 9.90(2.17~185.00) U=2587 0.238 HBV DNA (log10 IU/mL) 5.60(3.40~8.90) 5.70(3.40~8.90) 5.10(3.70~7.80) U=3692 0.030 HBeAg阳性[例(%)] 114 (52.3) 97(52.2) 17(53.1) χ2=1.040 >0.05 LSM (kPa) 16.1(4.2~60.4) 15.6(4.2~60.4) 19.1(4.8~41.0) U=2543 0.190 脾脏长度(mm) 113(69~194) 113(69~169) 115(86~194) U=2706 0.414 HAI评分[例(%)]1) 0.519 1~4分 25(11.5) 23(12.4) 2(6.3) 5~8分 118(54.1) 97(52.2) 21(65.6) 9~12分 68(31.2) 60(32.3) 8(25.0) 13~18分 7(3.2) 6(3.2) 1 (3.1) Ishak纤维评分[例(%)] χ2=1.358 0.621 5分 66(30.3) 58(31.2) 8(25.0) 6分 152(69.7) 128(68.8) 24(75.0) P-I-R分型[例(%)]1) 0.167 R 21(9.6) 15(8.1) 6(18.8) I 22(10.1) 19(10.2) 3(9.4) P 175(80.3) 152(81.7) 23(71.9) Laennec分级[例(%)] χ2=1.049 0.592 4A 23(10.6) 18(9.7) 5(15.6) 4B 41(18.8) 35(18.8) 6(18.8) 4C 154(70.6) 133(71.5) 21(65.6) 注:ETV, 恩替卡韦;PLC, 安慰剂; BJRG,鳖甲软肝片;HAI, 组织学活动指数。1)采用Fisher检验。 2.2 抗病毒治疗72周后患者的各指标比较
2组间P-I-R分型情况比较差异有统计学意义(P<0.001)(表 2)。
表 2 无HCC组和HCC组治疗后一般资料的比较Table 2. Comparison of general data after treatment between non-HCC group and HCC group项目 总体(n=218) 无HCC组(n=186) HCC组(n=32) 统计值 P值 BMI(kg/m2) 23.6(15.8~36.3) 23.6(15.8~36.3) 23.0(17.7~32.7) U=2333 0.773 Alb(g/L) 44.4(21.1~57.3) 44.3(21.1~57.3) 45.3(38.0~52.5) U=2447 0.336 TBil(μmol/L) 15.0(2.0~164.0) 15.1(2.0~164.0) 14.8(6.6~46.0) U=2209 0.661 ALT(U/L) 26.0(8.0~730.0) 26.0(9.0~730.0) 24.0(8.0~85.0) U=2477 0.638 AST(U/L) 33.9(8.0~803.0) 26.0(8.0~803.0) 27.0(13.0~68.0) U=2177 0.275 PLT(×109/L) 147.0(56.0~279.0) 150.0(56.0~279.0) 121.0(62.0~226.0) U=1552 0.061 AFP(ng/mL) 2.9(0.0~27.9) 2.9(0.0~27.9) 3.1(1.0~7.4) U=2420 0.967 HBV DNA(log10 IU/mL) N(N~4.11) N(N~4.11) N(N~3.39) U=2386 0.933 HBeAg阳性[例(%)] 98(45.0) 88(47.3) 10(31.3) χ2=0.508 0.135 LSM(kPa) 9.35(1.00~49.70) 9.50(1.00~49.70) 9.00(1.00~45.00) U=3305 0.318 LSM变化[例(%)]1) 0.867 减少 126(57.8) 106(57.0) 20(62.5) 稳定 80(36.7) 69(37.1) 11(34.4) 增加 12(5.5) 11(5.9) 1(3.1) 脾脏长度(mm) 109(24~183) 110(24~171) 108(80~183) U=3130 0.641 HAI评分[例(%)]1) 0.466 1~4分 0 0 0 5~8分 95(43.6) 84(45.2) 11(34.4) 9~12分 122(56.0) 101(54.3) 21(65.6) 13~18分 1(0.5) 1(0.5) 0 Ishak纤维评分[例(%)] χ2=2.533 0.06 5分 98(45.0) 89(47.8) 9(28.1) 6分 120(55.0) 97(52.2) 23(71.9) P-I-R分型[例(%)]1) <0.001 R 70(32.1) 69(37.1) 1(3.1) I 52(23.9) 49(26.3) 3(9.4) P 96(44.0) 68(36.6) 28(87.5) Laennec分级[例(%)] χ2=2.349 0.308 4A 33(15.1) 31(16.7) 2(6.3) 4B 71(32.6) 59(31.7) 12(37.5) 4C 114(52.3) 96(51.6) 18(56.3) 注:N,不可测出;1)采用Fisher检验。 2.3 治疗前后患者P-I-R分型和Laennec分级的比较
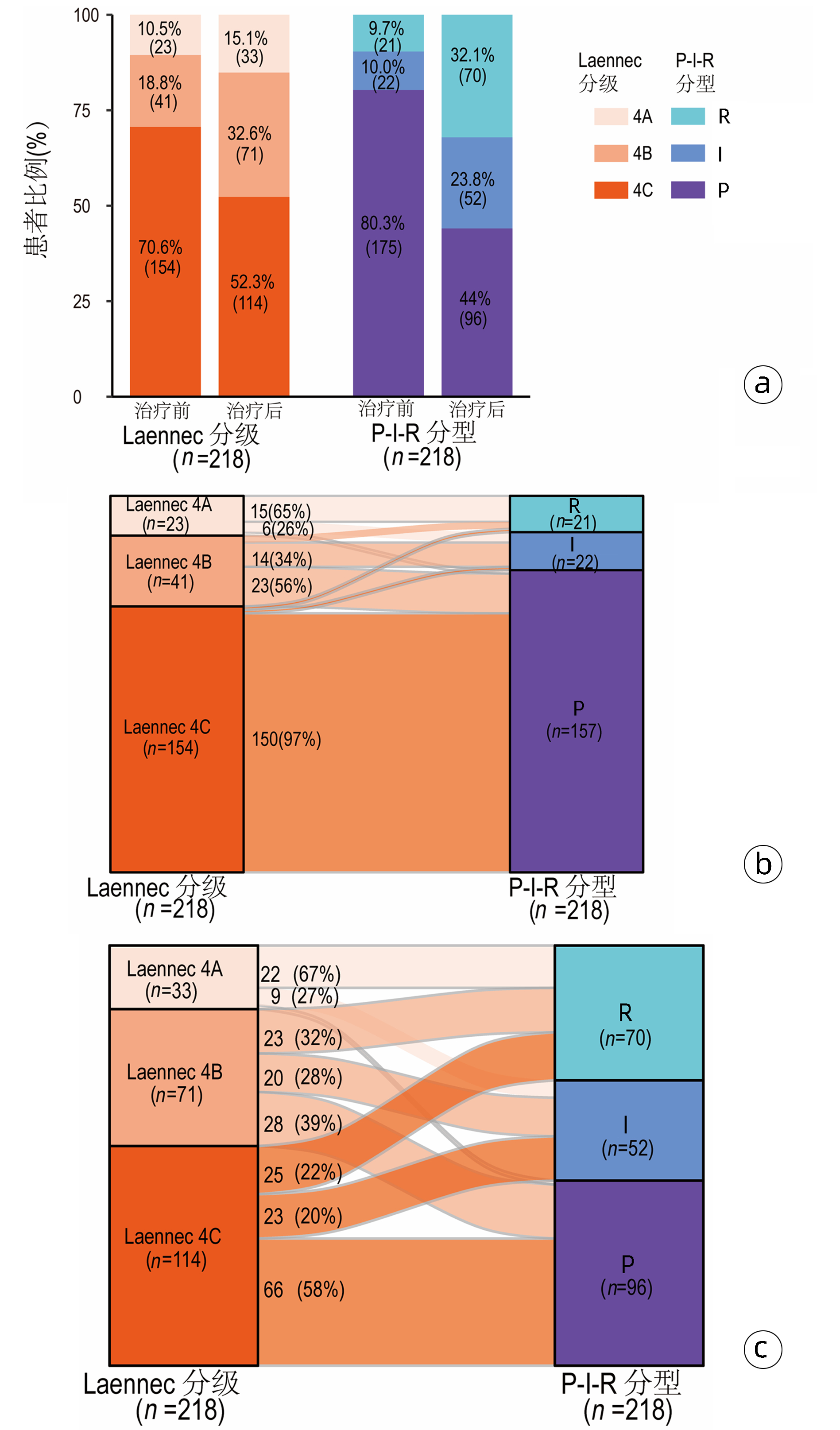
抗病毒治疗前后Laennec分级和P-I-R分型的分布均有统计学差异(P值均<0.001)(图 2a),治疗后两种评分方法均提示肝纤维化好转。
基线时,23例被评为Laennec分级为4A的患者中,P-I-R分型R组、I组、P组的比例分别为65%、26%、9%。41例评为Laennec分级4B的患者中,34%为P-I-R分型I组,56%为P组,另外10%为R组。154例Laennec分级为4C患者中,绝大多数(97%)为P-I-R分型P组(图 2b)。
抗病毒72周后,33例Laennec分级4A的患者中,大多数(67%)为P-I-R分型R组。71例Laennec分级为4B的患者中,被分流到P-I-R分型R组、I组、P组的比例较为平均,分别是32%、28%、39%。Laennec分级4C的114例患者中,大多数(58%)被评为P-I-R分型P组(图 2c)。
2.4 抗病毒治疗72周后的临床数据在P-I-R分型和Laennec分级中的分布差异
抗病毒治疗72周后,按照Laennec分级分为4A组(n=33)、4B组(n=71)、4C组(n=114)。3组间PLT、LSM、Ishak评分、HAI评分比较差异有统计学意义(P值均<0.05)(表 3)。
表 3 抗病毒治疗72周后的临床数据在Laennec分级中的分布差异Table 3. Differences in the distribution of clinical data in Laennec grading after 72 weeks of antiviral therapy项目 4A组(n=33) 4B组(n=71) 4C组(n=114) 统计值 P值 ALT(U/L) 28.0(9.0~83.0) 24.0(9.0~210.0) 27.0(8.0~121.0) H=0.569 0.481 AST(U/L) 26.0(18.0~49.0) 25.0(8.0~107.0) 27.4(13.0~177.0) H=4.119 0.635 PLT(×109/L) 161.0(56.0~258.0) 166.0(60.0~277.0) 129.0(69.0~279.0) H=36.429 <0.001 LSM(kPa) 6.7(3.1~28.9) 8.8(1.0~32.1) 11.8(1.0~49.7) H=13.983 0.004 LSM变化[例(%)]1) 0.848 减少 19(57.6) 42(59.2) 65(57.0) 稳定 14(42.4) 25(35.2) 41(36.0) 增加 0 4(5.6) 8(7.0) Ishak纤维化评分[例(%)] χ2=23.060 <0.001 5分 18(54.5) 46(64.8) 34(29.8) 6分 15(45.5) 25(35.2) 80(70.2) HAI评分[例(%)]1) <0.001 1~4分 21(63.6) 39(54.9) 35(30.7) 5~8分 12(36.4) 32(45.1) 78(68.4) 9~12分 0 0 1(0.9) 发生HCC[例(%)] χ2=2.349 0.512 是 2(6.1) 12(16.9) 18(15.8) 否 31(93.9) 59(83.1) 96(84.2) 注:1)采用Fisher检验。 抗病毒治疗72周后,按照P-I-R分型分为R组(n=70)、I组(n=52)和P组(n=96)。3组间PLT、LSM、Ishak评分、HAI评分、HCC发生情况比较,差异均有统计学意义(P值均<0.05)(表 4)。
表 4 抗病毒治疗72周后的临床数据在P-I-R分型中的分布差异Table 4. Differences of the distribution of clinical data of P-I-R types after 72 weeks of antiviral treatment项目 R组(n=70) I组(n=52) P组(n=96) 统计值 P值 ALT(U/L) 27(9~210) 26(9~108) 25(8~85) H=0.618 0.892 AST(U/L) 25.5(14.0~107.0) 26.0(8.0~76.0) 27.0(13.0~177.0) H=0.770 0.857 PLT(×109/L) 163(56~264) 146(66~260) 129(69~279) H=7.193 0.028 LSM(kPa) 8.65(3.10~28.90) 8.80(3.10~32.10) 10.60(1.00~49.70) H=6.238 0.045 LSM变化[例(%)]1) 0.995 减少 39(55.7) 31(59.6) 56(58.3) 稳定 28(40.0) 18(34.6) 34(35.4) 增加 3(4.3) 3(5.8) 6(6.3) Ishak纤维化评分[例(%)] χ2=7.986 <0.001 5分 36(51.4) 29(55.8) 33(34.4) 6分 34(48.6) 23(44.2) 63(65.6) HAI评分[例(%)]1) 0.002 1~4分 40(57.1) 26(50.0) 29(30.2) 5~8分 30(42.9) 26(50.0) 66(68.8) 9~12分 0 0 1(1.0) 发生HCC[例(%)]1) <0.001 是 1(1.4) 3(5.8) 28(29.2) 否 69(98.6) 49(94.2) 68(70.8) 注:1)采用Fisher检验。 P-I-R分型P组和Laennec分级4C组的患者明显具有更高水平的LSM值(图 3a)和更低PLT水平(图 3b),也表现出明显更高的Ishak纤维化评分(Ishak≥5)和HAI评分(图 3c、d)。
2.5 P-I-R分型预测乙型肝炎肝硬化患者发生HCC的风险
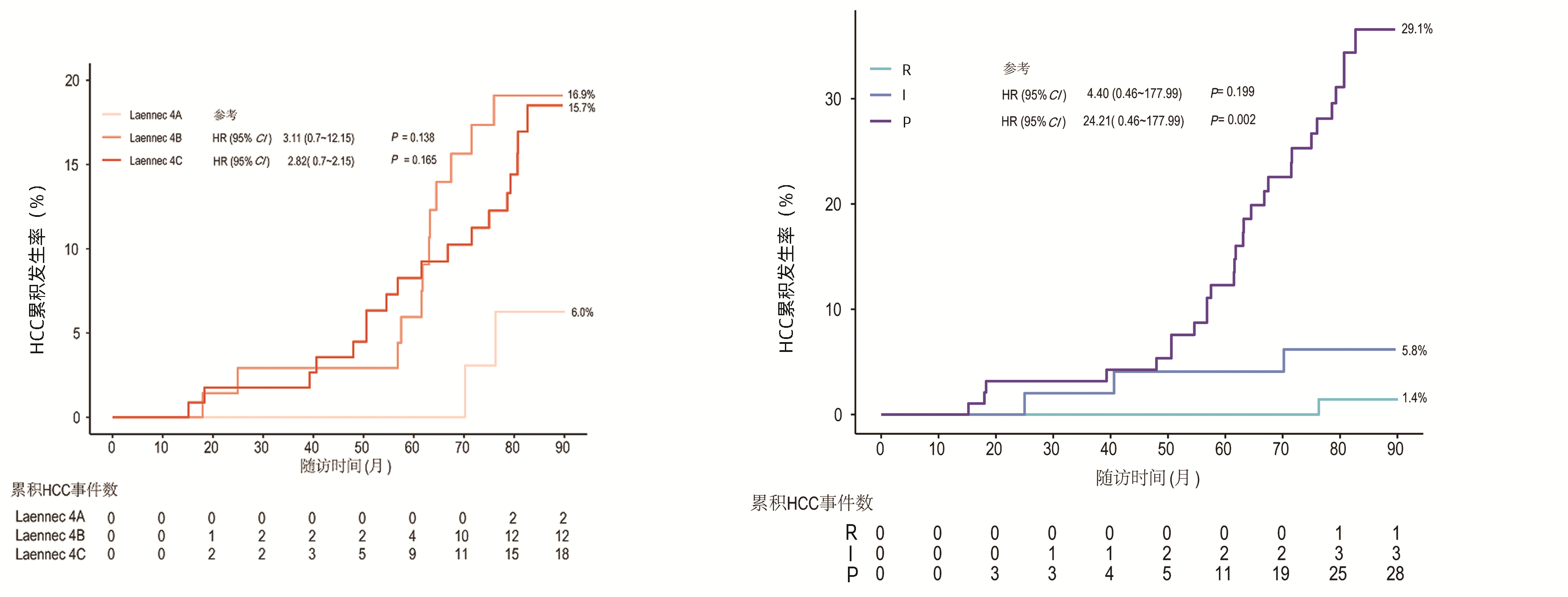
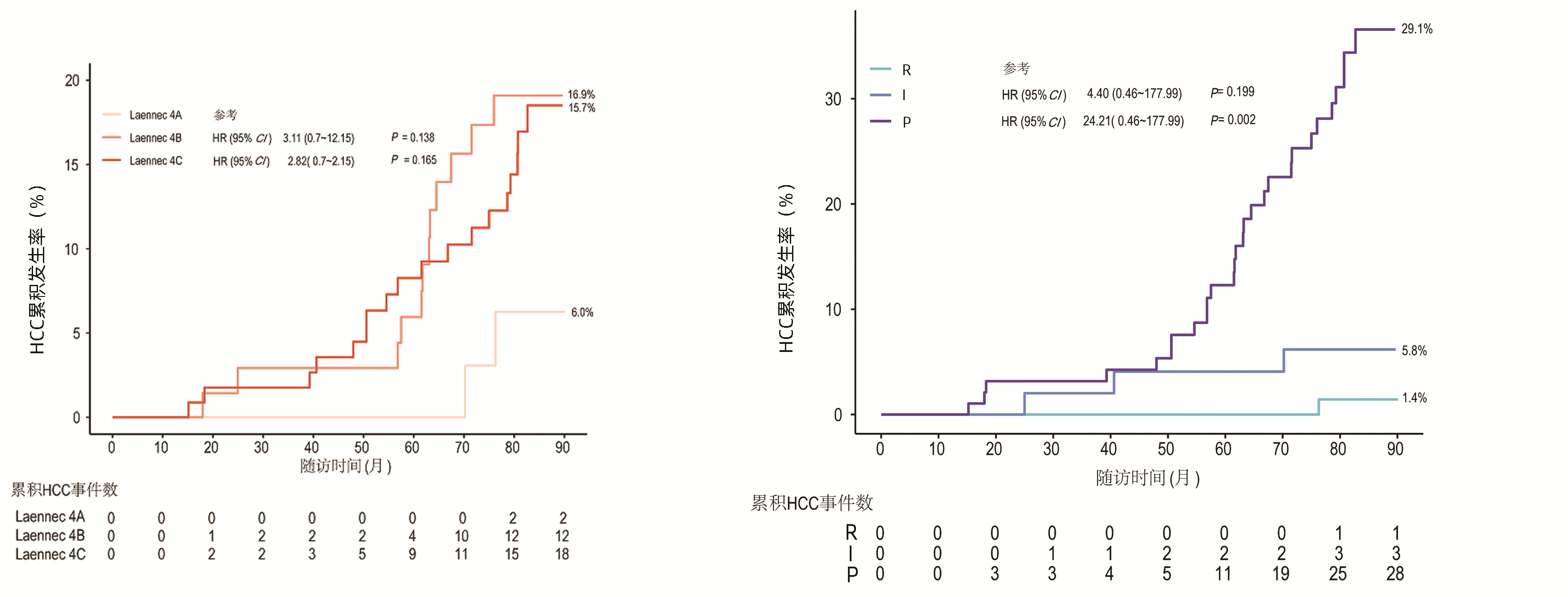
中位随访时间为83.0(12.9~90.1)个月。P-I-R评分患者随访时间相似(R、I、P组的随访中位数均为83个月)。Laennec分级的患者随访时间相似(4A、4B、4C组的随访中位数均为83个月)。32例发生了HCC。总体人群1、3、5和7年HCC累积发病率分别为0、1.8%、6.0%和14.7%。P-I-R分型(治疗后)R组、I组、P组的患者1、3、5、7年HCC累积发病率分别为0、0、0、1.4%, 0、1.9%、3.8%、5.8%, 0、3.1%、11.4%、29.1%。此外,Laennec分级4A、4B、4C的患者1、3、5、7年HCC累积发病率分别为0、0、0、6.0%, 0、2.8%、5.6%、16.9%, 0、1.8%、7.9%、15.7%。
对可能影响HCC发生的临床及病理特征进行单变量和多变量回归分析,结果显示,在单因素分析中,P-I-R分型和Ishak纤维化评分与HCC的发生有关;而Laennec分级与HCC的发生率无关(表 5, 图 4)。经调整其他因素后,P-I-R分型仍然是HCC发生的独立预测指标(P组vs R组:HR=12.69,95%CI: 4.63~34.80, P=0.002)。
表 5 单因素分析HCC发生的影响因素Table 5. Predictors of HCC occurrence by univariate analysis变量 HR(95%CI) P值 P-I-R分型 <0.001 R 1.00 I 4.40(0.46~177.99) 0.199 P 24.21(0.46~177.99) 0.002 Ishak纤维化评分(6 vs 5) 2.18(1.01~4.72) 0.047 PLT(>146.5 vs ≤146.5)×109 /L 0.59(0.29~1.21) 0.153 Ishak纤维化评分改变 0.411 好转 1.00 无变化 2.02(0.70~5.81) 0.191 进展 2.15(0.48~9.60) 0.317 治疗方式(ETV+PLC vs ETV+BJRG) 0.50(0.24~1.05) 0.068 Laennec组织学病理分级 0.327 4A 1.00 4B 3.11(0.70~12.15) 0.138 4C 2.82(0.70~12.15) 0.165 年龄(≥43岁vs<43岁) 1.01(0.97~1.05) 0.674 饮酒史(是vs否) 1.91(0.88~4.13) 0.100 性别(男vs女) 0.81(0.37~1.81) 0.615 HBV DNA(检测到vs未检测到) 1.06(0.32~3.49) 0.921 TBil(>15 mmol/L vs ≤15 mmol/L) 0.85(0.42~1.70) 0.644 BMI(>23.55 kg/m2 vs ≤23.55 kg/m2) 0.75(0.37~1.51) 0.418 AST(>26 U/L vs ≤26 U/L) 1.51(0.75~3.03) 0.249 AFP(>2.94 ng/mL vs ≤2.94 ng/mL) 1.57(0.77~3.17) 0.212 ALT(>26 U/L vs ≤26 U/L) 0.81(0.40~1.63) 0.561 LSM变化(kPa) 0.789 稳定 1.00 减少 1.25(0.61~2.55) 0.548 3. 讨论
本研究发现在传统乙型肝炎抗病毒治疗后,Ishak纤维化评分不能评价的(Ishak纤维化评分无变化率为69%)治疗前后均为肝硬化(Ishak纤维化评分≥5)的人群中,P-I-R分型和Laennec分级均能够反映患者抗病毒治疗后的病理特征。治疗后P-I-R评分对肝纤维化动态改变辨别能力优于Laennec分级。另外,本研究最重要的发现是:P-I-R分型可预测乙型肝炎肝硬化患者抗病毒治疗后发生HCC的风险。
乙型肝炎肝硬化人群HCC的发生与年龄、性别、肝硬化是否处于失代偿期、肝脏炎症是否持续等因素相关[18], 但在该研究中,除组织学指标P-I-R分型和Ishak纤维化评分外,只看到基线时的PLT水平、HBV DNA在有无HCC中是不同的。这或许跟本研究所纳入人群肝脏失代偿期统计数据较少、得到有效抗病毒治疗有关。目前关于肝纤维化的消退(包括Ishak纤维化评分)是否可降低HCC发生率的研究较少。但是,P-I-R分型是基于纤维间隔的形态。半定量和全定量研究[19-20]表明,纤维间隔宽度、结节大小等组织学参数与具有预测肝硬化预后能力的肝静脉压力梯度高度相关,纤维间隔宽度可作为临床显著性门静脉高压症(门静脉压力梯度≥10 mmHg)的独立预测因子。另有试验[21-22]可直接证实纤维间隔与临床终点事件的相关性。Laennec分级是基于METAVIR的亚分类,根据纤维间隔宽度和结节大小将肝硬化(METAVIR 4级)细分为4A、4B和4C亚型,研究[13-14]发现Laennec分级能够预测肝病相关事件(失代偿性肝硬化和HCC)。但本研究表明,P-I-R分型可用于评估抗病毒治疗后患者HCC发生的风险,而治疗后Laennec分级分级则不能。造成这种差异的原因可能有:(1)本研究人群均为乙型肝炎肝硬化患者,而前期研究[13-14]主要研究人群为慢性丙型肝炎肝硬化;(2)Laennec肝硬化亚分类的依据为纤维间隔宽度和结节大小,已有试验[23]证实纤维间隔宽度与HCC发生有关,而结节大小与HCC发生并不相关。
通过分析P-I-R分型和Laennec分级提供的组织学信息,可知两种评分系统都能反应患者抗病毒治疗后病理特征,这与研究[15]中P-I-R分型能够反应治疗前后因Ishak纤维化评分稳定不变而不能反应出组织学变化的说法是一致的。在该研究中,P-I-R分型相比Laennec分级能够更准确地反映组织学的变化趋势,这一区别与两个评分系统的开发过程有关——P-I-R分型是基于Wanless对“肝修复复合体”中纤维间隔的描述[24],将纤维间隔的动态变化作为新的组织学特征纳入到纤维分型中,根据不同纤维间隔的比例,将肝纤维化分为P型、I型和R型。
本研究也存在一些局限性。首先,肝纤维化半定量评价系统相对于胶原全定量的病理学评估方法来说评估更加主观[25],存在观察者间和观察者内的误差[7]。在本研究中,已通过控制不一致性因素将观察者间和观察者内的误差降到最低,包括收集并评价标本质量以保证组织学评价,由2名有经验的病理学家在对临床资料不知情时独立评价等。第三,本研究中由于肝脏相关事件的发生病例较少,因此没有对肝脏相关事件做进一步的相关分析。第四,由于本研究主要评估乙型肝炎肝硬化患者抗病毒治疗肝纤维化和发生肝癌的风险,因此本研究结论可能不适用于HCV、NASH或其他肝病病因的患者。第五,入选的患者局限于亚洲患者,这可能限制了结论在其他种族中的推广。
在未来的研究中,需要更进一步探讨P-I-R分型和Laennec分级是否与患者抗病毒治疗后肝脏相关事件的发生有关。其次,应开展能够提供与P-I-R分型相关的同期经颈静脉肝静脉楔形压测量或瞬时弹性成像结果的研究,验证P-I-R评分与临床结局和临床预后价值的关系。最后,对非洲、欧洲患者和患有HCV、NASH或其他病因性肝病的患者进行研究,以扩大结论的适用性。
综上所述,P-I-R分型和Laennec分级能反映乙型肝炎肝硬化患者抗病毒治疗前后肝纤维化的特征,其中P-I-R分型可评估抗病毒治疗后肝纤维化的动态改变。此外,P-I-R分型可预测乙型肝炎肝硬化患者抗病毒治疗后发生HCC的风险。因此,本研究可能为抗病毒治疗后HBV感染患者的最佳管理策略提供循证医学证据。同时,也可为进一步筛查HCC高风险人群提供坚实的理论依据。
-
表 1 无HCC组和HCC组基线资料的比较
Table 1. Comparison of baseline data between the non-HCC group and the HCC group
项目 总体(n=218) 无HCC组(n=186) HCC组(n=32) 统计值 P值 治疗方式[例(%)] χ2=0.476 0.097 ETV+PLC 117(53.7) 95 (51.1) 22(68.8) ETV+BJRG 101(46.3) 91(48.9) 10 (31.3) 年龄(岁) 46.0(19.0~68.0) 46.0(19.0~68.0) 47.0(34.0~63.0) U=2833 0.664 男性[例(%)] 157(72.0) 133(71.5) 24(75.0) χ2=0.837 0.846 饮酒史[例(%)] 39(17.9) 30(16.1) 9(28.1) χ2=2.207 0.166 BMI(kg/m2) 23.6(15.8~36.3) 23.7(15.8~36.3) 23.0(17.7~32.7) U=3070 0.777 Alb(g/L) 41.0(26.9~50.0) 41.0 (26.9~50.0) 42.0(30.4~48.0) U=2935 0.903 TBil(μmol/L) 15.2(5.0~149.0) 14.4(5.0~149.0) 17.4(6.1~47.9) U=2348 0.057 ALT(U/L) 50.5(10.0~1370.0) 49.0(10.0~1370.0) 66.5(13.0~854.0) U=2473 0.128 AST(U/L) 46.0(17.0~836.0) 45.0(17.0~836.0) 55.0(20.0~635.0) U=2415 0.089 PLT(×109/L) 125.0(45.0~277.0) 130.0(45.0~277.0) 116.0(50.0~223.0) U=3652 0.040 AFP(ng/mL) 7.00(0.91~200.00) 7.00(0.91~200.00) 9.90(2.17~185.00) U=2587 0.238 HBV DNA (log10 IU/mL) 5.60(3.40~8.90) 5.70(3.40~8.90) 5.10(3.70~7.80) U=3692 0.030 HBeAg阳性[例(%)] 114 (52.3) 97(52.2) 17(53.1) χ2=1.040 >0.05 LSM (kPa) 16.1(4.2~60.4) 15.6(4.2~60.4) 19.1(4.8~41.0) U=2543 0.190 脾脏长度(mm) 113(69~194) 113(69~169) 115(86~194) U=2706 0.414 HAI评分[例(%)]1) 0.519 1~4分 25(11.5) 23(12.4) 2(6.3) 5~8分 118(54.1) 97(52.2) 21(65.6) 9~12分 68(31.2) 60(32.3) 8(25.0) 13~18分 7(3.2) 6(3.2) 1 (3.1) Ishak纤维评分[例(%)] χ2=1.358 0.621 5分 66(30.3) 58(31.2) 8(25.0) 6分 152(69.7) 128(68.8) 24(75.0) P-I-R分型[例(%)]1) 0.167 R 21(9.6) 15(8.1) 6(18.8) I 22(10.1) 19(10.2) 3(9.4) P 175(80.3) 152(81.7) 23(71.9) Laennec分级[例(%)] χ2=1.049 0.592 4A 23(10.6) 18(9.7) 5(15.6) 4B 41(18.8) 35(18.8) 6(18.8) 4C 154(70.6) 133(71.5) 21(65.6) 注:ETV, 恩替卡韦;PLC, 安慰剂; BJRG,鳖甲软肝片;HAI, 组织学活动指数。1)采用Fisher检验。 表 2 无HCC组和HCC组治疗后一般资料的比较
Table 2. Comparison of general data after treatment between non-HCC group and HCC group
项目 总体(n=218) 无HCC组(n=186) HCC组(n=32) 统计值 P值 BMI(kg/m2) 23.6(15.8~36.3) 23.6(15.8~36.3) 23.0(17.7~32.7) U=2333 0.773 Alb(g/L) 44.4(21.1~57.3) 44.3(21.1~57.3) 45.3(38.0~52.5) U=2447 0.336 TBil(μmol/L) 15.0(2.0~164.0) 15.1(2.0~164.0) 14.8(6.6~46.0) U=2209 0.661 ALT(U/L) 26.0(8.0~730.0) 26.0(9.0~730.0) 24.0(8.0~85.0) U=2477 0.638 AST(U/L) 33.9(8.0~803.0) 26.0(8.0~803.0) 27.0(13.0~68.0) U=2177 0.275 PLT(×109/L) 147.0(56.0~279.0) 150.0(56.0~279.0) 121.0(62.0~226.0) U=1552 0.061 AFP(ng/mL) 2.9(0.0~27.9) 2.9(0.0~27.9) 3.1(1.0~7.4) U=2420 0.967 HBV DNA(log10 IU/mL) N(N~4.11) N(N~4.11) N(N~3.39) U=2386 0.933 HBeAg阳性[例(%)] 98(45.0) 88(47.3) 10(31.3) χ2=0.508 0.135 LSM(kPa) 9.35(1.00~49.70) 9.50(1.00~49.70) 9.00(1.00~45.00) U=3305 0.318 LSM变化[例(%)]1) 0.867 减少 126(57.8) 106(57.0) 20(62.5) 稳定 80(36.7) 69(37.1) 11(34.4) 增加 12(5.5) 11(5.9) 1(3.1) 脾脏长度(mm) 109(24~183) 110(24~171) 108(80~183) U=3130 0.641 HAI评分[例(%)]1) 0.466 1~4分 0 0 0 5~8分 95(43.6) 84(45.2) 11(34.4) 9~12分 122(56.0) 101(54.3) 21(65.6) 13~18分 1(0.5) 1(0.5) 0 Ishak纤维评分[例(%)] χ2=2.533 0.06 5分 98(45.0) 89(47.8) 9(28.1) 6分 120(55.0) 97(52.2) 23(71.9) P-I-R分型[例(%)]1) <0.001 R 70(32.1) 69(37.1) 1(3.1) I 52(23.9) 49(26.3) 3(9.4) P 96(44.0) 68(36.6) 28(87.5) Laennec分级[例(%)] χ2=2.349 0.308 4A 33(15.1) 31(16.7) 2(6.3) 4B 71(32.6) 59(31.7) 12(37.5) 4C 114(52.3) 96(51.6) 18(56.3) 注:N,不可测出;1)采用Fisher检验。 表 3 抗病毒治疗72周后的临床数据在Laennec分级中的分布差异
Table 3. Differences in the distribution of clinical data in Laennec grading after 72 weeks of antiviral therapy
项目 4A组(n=33) 4B组(n=71) 4C组(n=114) 统计值 P值 ALT(U/L) 28.0(9.0~83.0) 24.0(9.0~210.0) 27.0(8.0~121.0) H=0.569 0.481 AST(U/L) 26.0(18.0~49.0) 25.0(8.0~107.0) 27.4(13.0~177.0) H=4.119 0.635 PLT(×109/L) 161.0(56.0~258.0) 166.0(60.0~277.0) 129.0(69.0~279.0) H=36.429 <0.001 LSM(kPa) 6.7(3.1~28.9) 8.8(1.0~32.1) 11.8(1.0~49.7) H=13.983 0.004 LSM变化[例(%)]1) 0.848 减少 19(57.6) 42(59.2) 65(57.0) 稳定 14(42.4) 25(35.2) 41(36.0) 增加 0 4(5.6) 8(7.0) Ishak纤维化评分[例(%)] χ2=23.060 <0.001 5分 18(54.5) 46(64.8) 34(29.8) 6分 15(45.5) 25(35.2) 80(70.2) HAI评分[例(%)]1) <0.001 1~4分 21(63.6) 39(54.9) 35(30.7) 5~8分 12(36.4) 32(45.1) 78(68.4) 9~12分 0 0 1(0.9) 发生HCC[例(%)] χ2=2.349 0.512 是 2(6.1) 12(16.9) 18(15.8) 否 31(93.9) 59(83.1) 96(84.2) 注:1)采用Fisher检验。 表 4 抗病毒治疗72周后的临床数据在P-I-R分型中的分布差异
Table 4. Differences of the distribution of clinical data of P-I-R types after 72 weeks of antiviral treatment
项目 R组(n=70) I组(n=52) P组(n=96) 统计值 P值 ALT(U/L) 27(9~210) 26(9~108) 25(8~85) H=0.618 0.892 AST(U/L) 25.5(14.0~107.0) 26.0(8.0~76.0) 27.0(13.0~177.0) H=0.770 0.857 PLT(×109/L) 163(56~264) 146(66~260) 129(69~279) H=7.193 0.028 LSM(kPa) 8.65(3.10~28.90) 8.80(3.10~32.10) 10.60(1.00~49.70) H=6.238 0.045 LSM变化[例(%)]1) 0.995 减少 39(55.7) 31(59.6) 56(58.3) 稳定 28(40.0) 18(34.6) 34(35.4) 增加 3(4.3) 3(5.8) 6(6.3) Ishak纤维化评分[例(%)] χ2=7.986 <0.001 5分 36(51.4) 29(55.8) 33(34.4) 6分 34(48.6) 23(44.2) 63(65.6) HAI评分[例(%)]1) 0.002 1~4分 40(57.1) 26(50.0) 29(30.2) 5~8分 30(42.9) 26(50.0) 66(68.8) 9~12分 0 0 1(1.0) 发生HCC[例(%)]1) <0.001 是 1(1.4) 3(5.8) 28(29.2) 否 69(98.6) 49(94.2) 68(70.8) 注:1)采用Fisher检验。 表 5 单因素分析HCC发生的影响因素
Table 5. Predictors of HCC occurrence by univariate analysis
变量 HR(95%CI) P值 P-I-R分型 <0.001 R 1.00 I 4.40(0.46~177.99) 0.199 P 24.21(0.46~177.99) 0.002 Ishak纤维化评分(6 vs 5) 2.18(1.01~4.72) 0.047 PLT(>146.5 vs ≤146.5)×109 /L 0.59(0.29~1.21) 0.153 Ishak纤维化评分改变 0.411 好转 1.00 无变化 2.02(0.70~5.81) 0.191 进展 2.15(0.48~9.60) 0.317 治疗方式(ETV+PLC vs ETV+BJRG) 0.50(0.24~1.05) 0.068 Laennec组织学病理分级 0.327 4A 1.00 4B 3.11(0.70~12.15) 0.138 4C 2.82(0.70~12.15) 0.165 年龄(≥43岁vs<43岁) 1.01(0.97~1.05) 0.674 饮酒史(是vs否) 1.91(0.88~4.13) 0.100 性别(男vs女) 0.81(0.37~1.81) 0.615 HBV DNA(检测到vs未检测到) 1.06(0.32~3.49) 0.921 TBil(>15 mmol/L vs ≤15 mmol/L) 0.85(0.42~1.70) 0.644 BMI(>23.55 kg/m2 vs ≤23.55 kg/m2) 0.75(0.37~1.51) 0.418 AST(>26 U/L vs ≤26 U/L) 1.51(0.75~3.03) 0.249 AFP(>2.94 ng/mL vs ≤2.94 ng/mL) 1.57(0.77~3.17) 0.212 ALT(>26 U/L vs ≤26 U/L) 0.81(0.40~1.63) 0.561 LSM变化(kPa) 0.789 稳定 1.00 减少 1.25(0.61~2.55) 0.548 -
[1] D'AMICO G, MORABITO A, D'AMICO M, et al. Clinical states of cirrhosis and competing risks[J]. J Hepatol, 2018, 68(3): 563-576. DOI: 10.1016/j.jhep.2017.10.020. [2] HYTIROGLOU P, THEISE ND. Regression of human cirrhosis: an update, 18 years after the pioneering article by Wanless et al[J]. Virchows Arch, 2018, 473(1): 15-22. DOI: 10.1007/s00428-018-2340-2. [3] CHANG TT, LIAW YF, WU SS, et al. Long-term entecavir therapy results in the reversal of fibrosis/cirrhosis and continued histological improvement in patients with chronic hepatitis B[J]. Hepatology, 2010, 52(3): 886-893. DOI: 10.1002/hep.23785. [4] SCHIFF ER, LEE SS, CHAO YC, et al. Long-term treatment with entecavir induces reversal of advanced fibrosis or cirrhosis in patients with chronic hepatitis B[J]. Clin Gastroenterol Hepatol, 2011, 9(3): 274-276. DOI: 10.1016/j.cgh.2010.11.040. [5] RAMACHANDRAN P, IREDALE JP, FALLOWFIELD JA. Resolution of liver fibrosis: basic mechanisms and clinical relevance[J]. Semin Liver Dis, 2015, 35(2): 119-131. DOI: 10.1055/s-0035-1550057. [6] SINGAL AG, LIM JK, KANWAL F. AGA clinical practice update on interaction between oral direct-acting antivirals for chronic hepatitis C infection and hepatocellular carcinoma: Expert review[J]. Gastroenterology, 2019, 156(8): 2149-2157. DOI: 10.1053/j.gastro.2019.02.046. [7] ROCKEY DC, CALDWELL SH, GOODMAN ZD, et al. Liver biopsy[J]. Hepatology, 2009, 49(3): 1017-1044. DOI: 10.1002/hep.22742. [8] KNODELL RG, ISHAK KG, BLACK WC, et al. Formulation and application of a numerical scoring system for assessing histological activity in asymptomatic chronic active hepatitis[J]. Hepatology, 1981, 1(5): 431-435. DOI: 10.1002/hep.1840010511. [9] BATTS KP. Acute and chronic hepatic allograft rejection: pathology and classification[J]. Liver Transpl Surg, 1999, 5(4 Suppl 1): S21-S29. DOI: 10.1053/JTLS005s00021. [10] SCHEUER PJ. Classification of chronic viral hepatitis: a need for reassessment[J]. J Hepatol, 1991, 13(3): 372-374. DOI: 10.1016/0168-8278(91)90084-o. [11] LUDWIG J. The nomenclature of chronic active hepatitis: an obituary[J]. Gastroenterology, 1993, 105(1): 274-278. DOI: 10.1016/0016-5085(93)90037-d. [12] WANG W, LI J, PAN R, et al. Association of the Laennec staging system with degree of cirrhosis, clinical stage and liver function[J]. Hepatol Int, 2015, 9(4): 621-626. DOI: 10.1007/s12072-015-9648-7. [13] KIM MY, CHO MY, BAIK SK, et al. Histological subclassification of cirrhosis using the Laennec fibrosis scoring system correlates with clinical stage and grade of portal hypertension[J]. J Hepatol, 2011, 55(5): 1004-1009. DOI: 10.1016/j.jhep.2011.02.012. [14] KIM SU, OH HJ, WANLESS IR, et al. The Laennec staging system for histological sub-classification of cirrhosis is useful for stratification of prognosis in patients with liver cirrhosis[J]. J Hepatol, 2012, 57(3): 556-563. DOI: 10.1016/j.jhep.2012.04.029. [15] SUN Y, ZHOU J, WANG L, et al. New classification of liver biopsy assessment for fibrosis in chronic hepatitis B patients before and after treatment[J]. Hepatology, 2017, 65(5): 1438-1450. DOI: 10.1002/hep.29009. [16] RONG G, CHEN Y, YU Z, et al. Synergistic effect of biejia-ruangan on fibrosis regression in patients with chronic hepatitis B treated with entecavir: A multicenter, randomized, double-blind, placebo-controlled trial[J]. J Infect Dis, 2022, 225(6): 1091-1099. DOI: 10.1093/infdis/jiaa266. [17] JI D, CHEN Y, BI J, et al. Entecavir plus Biejia-Ruangan compound reduces the risk of hepatocellular carcinoma in Chinese patients with chronic hepatitis B[J]. J Hepatol, 2022, 77(6): 1515-1524. DOI: 10.1016/j.jhep.2022.07.018. [18] FATTOVICH G, STROFFOLINI T, ZAGNI I, et al. Hepatocellular carcinoma in cirrhosis: incidence and risk factors[J]. Gastroenterology, 2004, 127(5 Suppl 1): S35-S50. DOI: 10.1053/j.gastro.2004.09.014. [19] NAGULA S, JAIN D, GROSZMANN RJ, et al. Histological-hemodynamic correlation in cirrhosis-a histological classification of the severity of cirrhosis[J]. J Hepatol, 2006, 44(1): 111-117. DOI: 10.1016/j.jhep.2005.07.036. [20] SETHASINE S, JAIN D, GROSZMANN RJ, et al. Quantitative histological-hemodynamic correlations in cirrhosis[J]. Hepatology, 2012, 55(4): 1146-1153. DOI: 10.1002/hep.24805. [21] ZIPPRICH A, GARCIA-TSAO G, ROGOWSKI S, et al. Prognostic indicators of survival in patients with compensated and decompensated cirrhosis[J]. Liver Int, 2012, 32(9): 1407-1414. DOI: 10.1111/j.1478-3231.2012.02830.x. [22] RIPOLL C, BAÑARES R, RINCÓN D, et al. Influence of hepatic venous pressure gradient on the prediction of survival of patients with cirrhosis in the MELD Era[J]. Hepatology, 2005, 42(4): 793-801. DOI: 10.1002/hep.20871. [23] JAIN D, SREENIVASAN P, INAYAT I, et al. Thick fibrous septa on liver biopsy specimens predict the development of decompensation in patients with compensated cirrhosis[J]. Am J Clin Pathol, 2021, 156(5): 802-809. DOI: 10.1093/ajcp/aqab024. [24] WANLESS IR, NAKASHIMA E, SHERMAN M. Regression of human cirrhosis. Morphologic features and the genesis of incomplete septal cirrhosis[J]. Arch Pathol Lab Med, 2000, 124(11): 1599-1607. DOI: 10.5858/2000-124-1599-ROHC. [25] HE ZY, WANG BQ, YOU H. Clinical application of quantitative assessment of liver fibrosis based on pathology and imaging technology[J]. J Clin Hepatol, 2018, 35(1): 20-23. DOI: 10.3969/j.issn.1001-5256.2018.01.003.何志颖, 王冰琼, 尤红. 基于病理和影像学的肝纤维化量化评估技术的临床应用[J]. 临床肝胆病杂志, 2018, 34(1): 20-23. DOI: 10.3969/j.issn.1001-5256.2018.01.003. 期刊类型引用(1)
1. 陆晟瑛,姚文亿,从云,张跃,李京,吴申,刘畅,陈挺松. ~(125)I粒子条治疗肝细胞癌合并门静脉癌栓伴恶性梗阻性黄疸回顾性分析. 临床军医杂志. 2024(06): 634-637 .  百度学术
百度学术其他类型引用(0)
-




 PDF下载 ( 3408 KB)
PDF下载 ( 3408 KB)


 下载:
下载:



 下载:
下载:


 百度学术
百度学术