微波消融联合全身化疗治疗复发性肝内胆管癌的有效性与安全性分析
DOI: 10.3969/j.issn.1001-5256.2023.07.015
Efficacy and safety of microwave ablation combined with systemic chemotherapy in treatment of recurrent intrahepatic cholangiocarcinoma
-
摘要:
目的 探讨微波消融(MWA)联合化疗与单独MWA治疗复发性肝内胆管癌(RICC)的有效性和安全性。 方法 采用回顾性队列研究方法。选取2014年1月—2021年3月内江市第二人民医院及西南医科大学附属医院接受MWA+ 化疗和单独MWA的RICC患者,收集入组患者的临床病理资料。计量资料两组间比较采用成组t检验,计数资料两组间比较采用χ2检验和Fisher精确检验。釆用Kaplan-Meier法计算无进展生存期(PFS)和总生存期(OS)。使用Log-rank检验方法比较生存差异。应用单因素和多因素Cox比例风险回归模型分析生存预后的危险因素。 结果 共筛选到106例RICC患者,其中MWA+化疗组55例,MWA组51例。至随访截止,MWA+化疗组的中位PFS为15.0个月(95%CI:14.5~15.5),MWA组中位PFS为13.4个月(95%CI:11.6~15.2),两组差异有统计学意义(χ2=9.624,P=0.002)。MWA+化疗组的中位OS为21.0个月(95%CI:20.0~21.8),MWA组中位OS为18.0个月(95%CI:16.3~19.7),两组差异有统计学意义(χ2=12.784,P<0.001)。Cox回归分析显示,肿瘤直径(HR=0.425, 95%CI:0.208~0.868,P=0.019;HR=0.299, 95%CI:0.121~0.739,P=0.009)、复发时间(HR=7.064, 95%CI:3.612~13.618,P<0.001;HR=2.341, 95%CI:1.072~5.113,P=0.033)及联合化疗(HR=0.138, 95%CI:0.069~0.276,P<0.001;HR=0.175, 95%CI:0.081~0.380,P<0.001)是RICC患者PFS和OS的独立影响因素。两组常见不良反应中,除血液学毒性发生率(χ2=12.524,P<0.001)外,其余不良反应发生率差异均无统计学意义(P值均>0.05)。 结论 与单独MWA相比,MWA+化疗可以改善RICC的预后,延长其PFS和OS,且副反应安全可控。肿瘤直径>5 cm、复发时间<1年、未联合全身化疗的患者预后不良。 Abstract:Objective To investigate the efficacy and safety of microwave ablation (MWA) combined with chemotherapy versus MWA alone in the treatment of recurrent intrahepatic cholangiocarcinoma (RICC). Methods A retrospective cohort study was conducted among the patients with RICC who received MWA+chemotherapy or MWA in The Second People's Hospital of Neijiang and The Affiliated Hospital of Southwest Medical University from January 2014 to March 2021, and their clinicopathological data were collected. The independent samples t-test was used for comparison of continuous data, and the chi-square test and the Fisher's exact test were used for comparison of categorical data. The Kaplan-Meier method was used to calculate progression-free survival (PFS) and overall survival (OS), and the Log-rank test was used for comparison of survival differences. Univariate and multivariate Cox proportional-hazards regression model analyses were used to investigate the risk factors for survival and prognosis. Results A total of 106 patients with RIC were enrolled, among whom there were 55 patients in the MWA+chemotherapy group and 51 in the MWA group. By the end of follow-up, the MWA+chemotherapy group had a median PFS of 15.0 months (95% confidence interval [CI]: 14.5-15.5), and the MWA group had a median PFS of 13.4 months (95%CI: 11.6-15.2), with a significant difference between the two groups (χ2=9.624, P=0.002). The MWA+chemotherapy group had a median OS of 21.0 months (95%CI: 20.0-21.8), and the MWA group had a median OS of 18.0 months (95%CI: 16.3-19.7), with a significant difference between the two groups (χ2=12.784, P < 0.001). The Cox regression analysis showed that tumor diameter (PFS: hazard ratio [HR]=0.425, 95%CI: 0.208-0.868, P=0.019; OS: HR=0.299, 95%CI: 0.121-0.739, P=0.009), time to recurrence (PFS: HR=7.064, 95%CI: 3.612-13.618, P < 0.001; OS: HR=2.341, 95%CI: 1.072-5.113, P=0.033), and combined chemotherapy (PFS: HR=0.138, 95%CI: 0.069-0.276, P < 0.001; OS: HR=0.175, 95%CI: 0.081-0.380, P < 0.001) were independent influencing factors for PFS and OS in patients with RICC. As for the common adverse reactions, there were no significant differences in the incidence rates of all adverse reactions except hematological toxicity (χ2=12.524, P < 0.001). Conclusion Compared with MWA alone, MWA combined with chemotherapy can improve the prognosis of RICC and prolong PFS and OS, with safe and controllable side effects. Patients with tumor diameter > 5 cm, time to recurrence < 1 year, and absence of systemic chemotherapy tend to have a poor prognosis. -
Key words:
- Cholangiocarcinoma /
- Ablation Techniques /
- Drug Therapy
-
肝内胆管癌(intrahepatic cholangiocarcinoma,ICC) 是起源于肝内胆管上皮细胞的一种恶性肿瘤,占原发性肝癌的5%~10%,其发病率正逐步上升[1]。近年来,ICC的系统治疗取得了巨大的进展[2],但根治性手术切除仍然是其主要治疗方式[3]。由于ICC的高度侵袭性,多数患者在术后1~2年内出现肿瘤复发,导致ICC行根治性肝切除术后的5年生存率仅为20%~40%[4-5]。尽管再次切除(repeat resection,RR)能够延长患者的总生存期(overall survival,OS)[6],但受限于残肝体积不足和多灶性复发等因素[7],仅有极少部分患者可获得再次手术的机会。图像引导的热消融是复发性ICC(recurrent intrahepatic cholangiocarcinoma,RICC)的另一种治疗方式,而微波消融(microwave ablation,MWA)因其对实体肿瘤优异的治疗效果而被广泛报道[8-9],具有瘤内温度更高、消融面积更大、操作时间更短等优势。有研究[10]报道,与RR相比较,MWA可获得类似的疗效且并发症更少。然而仅接受局部治疗的RICC患者,超过50%在1年内出现再次复发[11]。而系统治疗的联合可能有助于进一步加强局部治疗的疗效,从而获得长期的生存[12]。
基于ABC-02 Ⅲ期临床试验的里程碑结果,顺铂和吉西他滨的化疗方案成为不可切除和晚期ICC的标准一线治疗方案[13]。化疗作为术后辅助治疗也可改善部分ICC患者的预后[14]。对于RICC患者而言,化疗是一种潜在获益的治疗方式[11]。然而,目前少有关于MWA联合化疗和单独MWA对于RICC疗效比较的研究,本研究旨在阐明两种治疗方案的有效性及安全性。
1. 资料与方法
1.1 研究对象
选取2014年1月—2021年3月在内江市第二人民医院及西南医科大学附属医院接受根治性肝切除术的ICC患者213例。对RICC的治疗原则如下:入院后经多学科会诊制定治疗策略,对全身情况良好,Child-Pugh A或B级,孤立、少结节(2~3个结节)复发或肿瘤局限,预计再次根治术后残肝体积超过标准肝体积40%的患者推荐RR;对不能耐受手术,复发病灶数≤3个,肿瘤最大直径≤5 cm的患者推荐MWA或MWA联合化疗,对肿瘤最大直径>5 cm或富血供肿瘤患者推荐联合TACE治疗。具体治疗取决于患者的肝功能状态、全身状况、肿瘤相关情况等,并且在实施前征求患者和家属的知情同意。纳入标准:(1)根治性肝切除术后首次肝内复发;(2)肿瘤个数≤3个;(3)无大血管侵犯或肝外转移;(4) Child-Pugh A或B级;(5)东部肿瘤协作组(Eastern cooperative oncology group,ECOG)评分为0~1分,预期生存期>3个月。排除标准:(1)反复复发的ICC;(2) 严重的内科合并症,包括心、肺、肾功能障碍;(3)有严重的凝血障碍(即凝血酶原时间>25 s,凝血酶原活性<40%,血小板计数<50×109/L);(4)活动性严重感染。最终,共有106例接受了MWA或MWA+化疗的RICC患者被纳入本研究。
收集入组患者临床病理学资料:年龄、性别、并发症、肝硬化、乙型肝炎标志物、肝功能Child-Pugh分级、复发肿瘤直径、复发肿瘤数量、分化程度(以第一次切除时病理分化为准)和实验室检查(以首次复发后查血结果为准)、治疗方式、再次复发或转移的日期,以及最后一次随访的日期和状态。
1.2 治疗方法
1.2.1 MWA
治疗前患者行增强CT/MRI,通过超声或CT选择合适的穿刺路径,将一次性微波消融针插入肝脏中,并在超声或CT引导下放置到指定肿瘤部位。功率设置为50 W,消融时间约5 min。当肿瘤位于距皮肤表面不超过5 mm或邻近肠、胆囊或其他重要组织时,进行水分离。术毕退针过程中消融针道。术后发现有肿瘤残留时,再次消融。
1.2.2 化疗
患者在行MWA后的2周内开始化疗。以吉西他滨为基础的化疗方案33例,其中吉西他滨联合顺铂14例(1~4周期),吉西他滨联合奥沙利铂6例(2~4周期),吉西他滨联合替吉奥7例(1~4周期),吉西他滨单药化疗6例(3~4周期)。其余治疗方案包括,替吉奥单药化疗10例(3~6周期),卡培他滨单药化疗12例(2~8周期)。化疗剂量参照相关指南推荐,根据患者一般情况及耐受性进行适量调整[15]。
1.3 随访
随访截止时间为2022年6月。复查方式:胸腹盆CT或腹部MRI扫描。随访方式及时间与既往研究类似[16],患者在治疗结束后前1~3年每3~4个月进行一次复查,3年后每6个月进行一次复查。根据实体瘤疗效评价标准1.1版[17]对患者进行肿瘤评价。研究终点为患者的OS和无进展生存期(progression free survival,PFS)。PFS为从接受MWA开始,到观察到肿瘤进展的时间或最后一次随访日期(未发现复发或丢失)。OS为手术后日期至死亡日期(未达到终点者,计算至末次随访日期)。对于再次复发患者,根据患者不同复发模式,体能状况及肝功能状态制定后续治疗方式。
1.4 统计学方法
采用IBM SPSS 26.0统计软件对数据进行统计分析。计量资料以x±s表示,两组间比较采用成组t检验,计数资料两组间比较采用χ2检验和Fisher精确检验。釆用Kaplan-Meier法计算PFS和OS。使用Log-rank检验比较生存差异。应用单因素和多因素Cox比例风险回归模型分析生存预后的危险因素。P<0.05表示差异有统计学意义。
2. 结果
2.1 一般资料
共纳入106例患者,MWA+全身化疗组55例,MWA组51例,其中76例男性和30例女性,入组患者的中位年龄为54岁。两组基线资料比较均无显著差异(P值均>0.05)(表 1)。
表 1 两组基线资料比较Table 1. Comparison of baseline data between the two groups项目 MWA+化疗(n=55) MWA(n=51) 统计值 P值 年龄(岁) 55.008±8.829 55.099±9.070 t=0.211 0.958 男性[例(%)] 39(70.9) 37(72.5) χ2=0.035 0.851 HBV感染 33(60.0) 27(52.9) χ2=0.537 0.464 肝硬化[例(%)] 36(65.5) 31(60.8) χ2=0.248 0.618 TBil(μmol/L) 41.025±13.796 37.208±12.988 t=1.465 0.146 AST(U/L) 65.891±23.122 73.093±24.721 t=-1.551 0.125 ALT(U/L) 69.755±24.965 79.076±35.183 t=-1.562 0.122 Alb(g/L) 38.684±4.069 38.882±3.806 t=-0.265 0.797 PT(s) 14.082±1.326 14.172±1.667 t=-0.310 0.757 INR 1.344±0.197 1.373±0.178 t=-0.806 0.421 CA19-9[例(%)] χ2=0.621 0.431 ≤37 U/mL 36(65.5) 37(72.5) >37 U/mL 19(34.5) 14(27.5) 术后复发TNM分期[例(%)] χ2=0.865 0.649 Ⅰ期 31(56.4) 26(51.0) Ⅱ期 17(30.9) 20(39.2) Ⅲ期 7(12.7) 5(9.8) 肿瘤直径[例(%)] χ2=0.907 0.119 ≤5 cm 35(63.6) 26(51.0) >5 cm 20(36.4) 25(49.0) 肿瘤个数[例(%)] χ2=0.794 0.268 1个 41(74.5) 34(66.7) 2~3个 14(25.5) 17(33.3) 首次切除时分化程度[例(%)] χ2=0.095 0.758 中高度 34(61.8) 33(64.7) 低度 21(38.2) 18(35.3) Child-Pugh分级[例(%)] χ2=0.484 0.486 A级 37(67.3) 31(60.8) B级 18(32.7) 20(39.2) 距初次切除后的复发时间[例(%)] χ2=2.358 0.125 ≤1年 33(60.0) 23(45.1) >1年 22(40.0) 28(54.9) 联合TACE[例(%)] χ2=0.028 0.867 是 38(69.1) 36(70.6) 否 17(30.9) 15(29.4) 2.2 生存情况
中位随访时间为16.0个月。截止随访结束,共有44例(41.5%)患者死亡,其中MWA+全身化疗组26例(24.5%),MWA组共有18例(17.0%),无治疗相关的死亡。44例死亡患者中35例(33.0%)因肝衰竭死亡,6例(5.7%)因多器官功能衰竭死亡,2例(1.9%)因消化道出血死亡,1例(0.9%)因脓毒血症死亡。
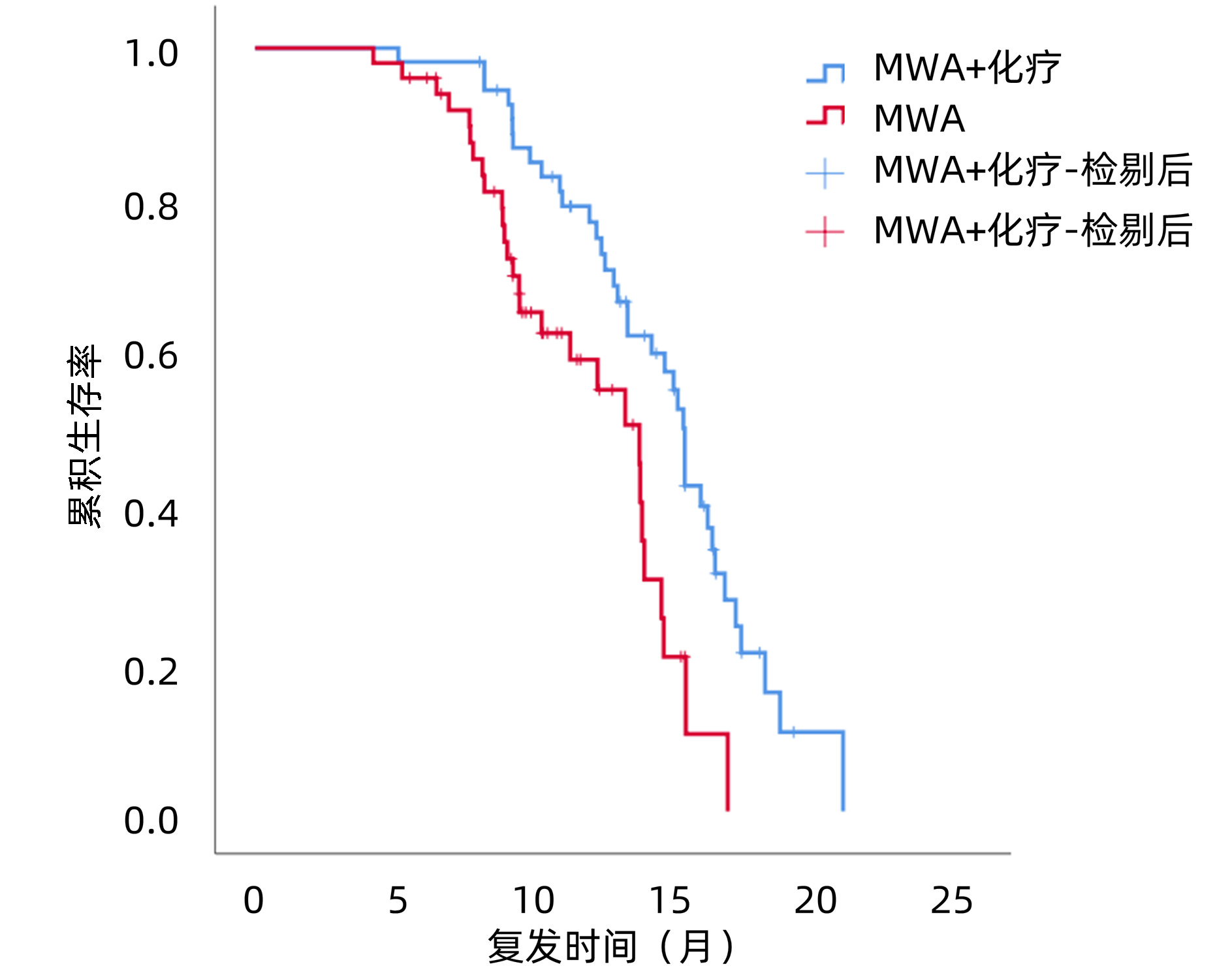
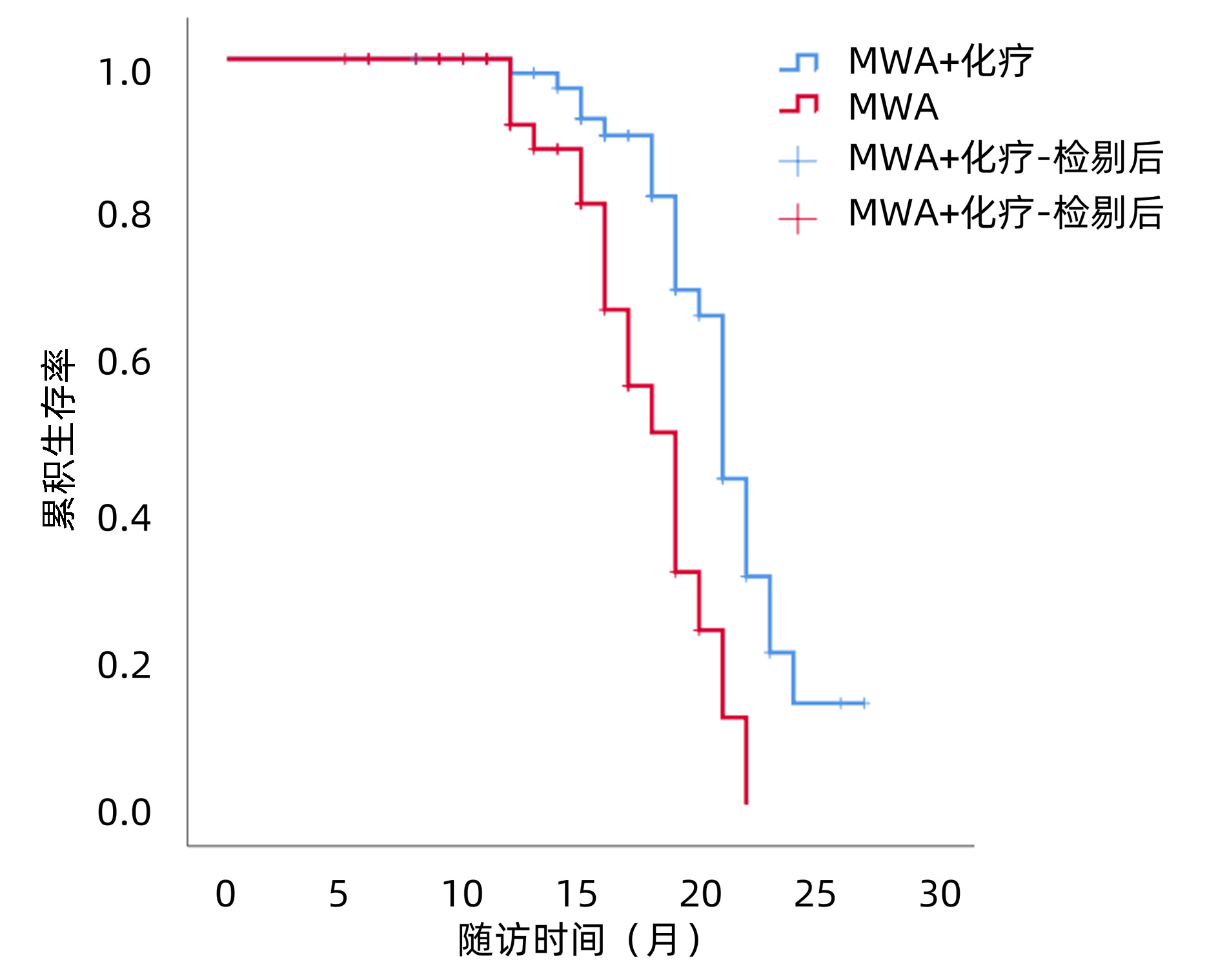
MWA+化疗组的中位PFS为15.0个月(95%CI:14.5~15.5),MWA组中位PFS为13.4个月(95%CI:11.6~15.2),两组差异有统计学意义(χ2=9.624,P=0.002)(图 1)。MWA+全身化疗组的中位OS为21.0个月(95%CI:20.0~21.8),MWA组中位OS为18.0个月(95%CI:16.3~19.7),两组差异有统计学意义(χ2=12.784,P<0.001)(图 2)。
2.3 预后影响因素分析
单因素分析结果显示TBil、Alb、CA19-9水平、肿瘤直径、分化程度、复发时间、联合化疗是RICC患者PFS的相关因素。将上述7个因素纳入Cox多因素分析显示,肿瘤直径(HR=0.425, 95%CI: 0.208~0.868,P=0.019)、复发时间(HR=7.064, 95%CI: 3.612~13.618, P<0.001)以及联合化疗(HR=0.138, 95%CI: 0.069~0.276, P<0.001)是RICC患者PFS的独立影响因素(表 2)。
表 2 RICC患者PFS的单因素分析Table 2. Univariate analysis of PFS in patients with RICC项目 HR P值 性别(男/女) 0.708(0.408~1.228) 0.219 年龄(>60/≤60岁) 1.243(0.729~2.120) 0.423 HBV(+)(是/否) 0.859(0.517~1.426) 0.557 TBil(>34.2 μmol/L/≤34.2 μmol/L) 2.498(1.497~4.169) 0.001 AST(>40 U/L/≤40 U/L) 0.754(0.343~1.657) 0.482 ALT(>40 U/L/≤40 U/L) 1.692(0.760~3.768) 0.198 Alb(>35 g/L/≤35 g/L) 1.930(1.061~3.512) 0.031 CA19-9(>37 ng/mL/≤37 ng/mL) 0.326(0.193~0.550) <0.001 TNM分期(≥Ⅱ期/Ⅰ期) 1.012(0.727~1.409) 0.943 肿瘤直径(>5 cm/≤5 cm) 0.353(0.212~0.588) <0.001 肿瘤个数(1个/2~3个) 0.778(0.453~1.335) 0.361 分化程度(中高/低) 0.472(0.287~0.775) 0.003 Child-Pugh分级(A/B) 1.165(0.690~1.969) 0.568 复发时间(>1年/≤1年) 4.286(2.505~7.331) <0.001 联合化疗(是/否) 0.437(0.255~0.747) 0.002 联合TACE(是/否) 0.756(0.446~1.281) 0.298 以OS为作为生存预后的结局指标,单因素分析结果显示,CA19-9水平、肿瘤直径、分化程度、复发时间、联合化疗是RICC患者OS的相关因素。将上述5个变量纳入Cox多因素分析后显示,肿瘤直径(HR=0.299, 95%CI: 0.121~0.739, P=0.009)、复发时间(HR=2.341, 95%CI: 1.072~5.113, P=0.033)以及联合化疗(HR=0.175, 95%CI: 0.081~0.380, P<0.001)是RICC患者OS的独立影响因素(表 3)。
表 3 RICC患者OS的单因素分析Table 3. Univariate analysis of OS in patients with RICC项目 HR P值 性别(男/女) 0.625(0.329~1.187) 0.151 年龄(>60岁/≤60岁) 0.753(0.578~2.134) 0.753 HBV(+)(是/否) 0.754(0.413~1.376) 0.357 TBil(>34.2 μmol/L/≤34.2 μmol/L) 1.570(0.855~2.883) 0.146 AST(>40 U/L/≤40 U/L) 0.912(0.357~2.332) 0.848 ALT(>40 U/L/≤40 U/L) 2.010(0.778~5.196) 0.150 Alb(>35.0 g/L/≤35.0 g/L) 1.429(0.658~3.106) 0.367 CA19-9>40 ng/mL(是/否) 0.412(0.224~0.756) 0.004 TNM分期(≥Ⅱ期/Ⅰ期) 1.188(0.652~2.166) 0.574 肿瘤直径(>5 cm/≤5 cm) 0.244(0.127~0.470) <0.001 肿瘤个数(1个/2~3个) 0.835(0.427~1.631) 0.598 分化程度(中高/低) 0.502(0.274~0.922) 0.026 Child-Pugh分级(A/B) 0.970(0.512~1.839) 0.927 复发时间(>1年/≤1年) 2.606(1.382~4.912) 0.003 联合化疗(是/否) 0.339(0.177~0.651) 0.001 联合TACE(是/否) 0.629(0.335~1.179) 0.148 2.4 不良反应
两组常见不良事件包括:血液学毒性、乏力、胃肠道反应、疼痛、肝功能异常、发热。其中,血液学毒性发生率差异具有统计学意义(χ2=12.524,P<0.05),经过药物减量和对症处理后好转。其余常见不良反应发生率差异无统计学意义(P值均>0.05)(表 4)。MWA+全身化疗组与MWA组发生3级及以上不良事件的概率分别为14.5%和7.8%,差异无统计学意义(P=0.277)。
表 4 两组不良反应比较Table 4. Comparison of complications between the two groups项目 MWA+化疗(n=55) MWA(n=51) χ2值 P值 所有级别 3~4级 所有级别 3~4级 血液学毒性[例(%)] 18(32.7) 2(3.6) 2(3.9) 0(0.0) 12.524 <0.001 乏力[例(%)] 22(40.0) 3(5.5) 16(31.4) 1(2.0) 0.856 0.355 胃肠道反应[例(%)] 21(38.2) 2(3.6) 11(21.6) 1(2.0) 3.465 0.063 疼痛[例(%)] 18(32.7) 0(0.0) 15(29.4) 2(3.9) 0.136 0.713 肝功能异常[例(%)] 16(29.1) 1(1.8) 10(19.6) 0(0.0) 1.286 0.257 发热[例(%)] 8(14.5) 0(0.0) 4(7.8) 0(0.0) 0.277 出血[例(%)] 1(1.8) 0(0.0) 2(3.9) 0(0.0) 0.607 腹水/胸水[例(%)] 2(3.6) 0(0.0) 4(7.8) 0(0.0) 0.425 血栓[例(%)] 1(1.8) 0(0.0) 0(0.0) 0(0.0) >0.05 3. 讨论
ICC术后易出现局部复发和淋巴道转移, 5年复发率超过70%, 其中大部分患者在术后1年内复发[18]。RICC的最佳治疗方法尚无定论,研究者进行了多种治疗方式的尝试,包括化疗、放疗、RR、热消融和经肝动脉化疗栓塞术等[19]。欧洲肝病学会关于ICC管理指南建议,肝内复发的情况下可以选择RR或者热消融治疗[10]。然而RR的实施不仅对外科技术提出了一定的挑战,也需要对患者残肝体积、肿瘤负荷、肿瘤位置进行严格筛选[20]。
MWA因其良好的安全性及有效性,已开展应用于RICC的治疗[21]。Xu等[10]纳入分析了121例RICC患者,MWA和RR后的5年生存率分别为23.7%和21.8%,3年无复发生存率分别为33.1%和30.6%,但MWA组的术后并发症明显低于RR组。然而作为局部治疗手段,MWA仍表现出一定的不足:对于肿瘤较大以及多肿瘤病灶可能消融不彻底,消融后边缘复发[22]。2019版美国临床肿瘤学会指南提出,系统性化疗与其他多种治疗方式联用可能是RICC潜在的治疗策略[23]。Yuan等[12]研究显示,接受系统联合局部治疗的RICC患者生存期高于仅接受单独局部治疗的患者,然而其中联合化疗患者仅有3例,需要进一步的探索分析其对预后的影响。
本研究结果显示,在MWA的基础上加用全身化疗不仅可以改善患者的PFS(15.0个月vs 13.4个月),还可以明显延长患者的OS(21.0个月vs 18.0个月)。在MWA的基础上联合化疗与RICC患者的PFS(HR=0.255,95%CI:0.138~0.472,P<0.001)和OS(HR=0.208,95%CI:0.098~0.439,P<0.001)呈正相关,因此可见联合治疗对生存改善更有益。与单独MWA治疗相比,其3级及以上不良事件的发生率未见显著差异,常见不良反应中仅有血液学毒性发生率明显增加,但通过药物减量和对症治疗后均可使症状得到缓解,表明联合治疗副反应相对可控。
值得关注的是,多因素分析结果显示,肿瘤直径、复发时间以及联合化疗同时是PFS和OS的独立影响因素。复发时间<1年与患者肿瘤生物学行为具有更强的侵袭性相关,往往复发病灶在首次手术前或术中可能已发生肝内微小转移或切缘癌残留,这类患者对多种治疗的反应较差[7]。Diaz-Gonzálezá等[24]的一项单中心的研究结果显示,肿瘤数量是决定经MWA治疗后ICC患者OS的独立危险因素。在本研究中,对于RICC患者,肿瘤数量与PFS和OS没有显著相关性,这可能是由原发性ICC和RICC的肿瘤异质性所致。消融的疗效取决于完全消融率,而肿瘤直径决定了能否完全消融。对于直径<3 cm的ICC,局部消融已经可以获得与二次手术相当的疗效[25]。但当肿瘤直径较大(>5 cm)时,单独消融的疗效并不肯定[26]。本研究结果也提示了肿瘤直径>5 cm时预后不良,这可能是未达到完全消融所致。另外,本研究中联合TACE与预后无显著相关性,分析原因可能是ICC多为乏血供肿瘤,传统的TACE治疗效果往往差强人意[27]。近年来,国内有相关研究报道采用直径较小(100~300 μm)的Calli Spheres载药微球治疗乏血供肝肿瘤能获得较好的疗效,其加载的多种化疗药物能持续有效杀伤肿瘤细胞[28]。这也为治疗肿瘤直径较大、乏血供的RICC提供了新的治疗思路。
系统性化疗能够通过杀灭残留肿瘤细胞以及微转移灶而改善患者的预后[29],但目前指南中暂无关于RICC标准的化疗方案,往往借鉴于晚期ICC的化疗方案,包括吉西他滨、5-氟尿嘧啶、卡培他滨、替吉奥、奥沙利铂、顺铂等单药或联合治疗。基于BILCAP试验结论,卡培他滨被推荐作为ICC切除后的首选化疗药物[2]。一项多中心的临床试验[30]结果显示,术后接受吉西他滨联合顺铂化疗的ICC患者预后优于观察组的患者,另一项研究[31]指出吉西他滨联合奥沙利铂方案作为ICC术后辅助治疗并未显示出临床获益,然而对于术后淋巴结阳性和切缘阳性的高危患者仍然推荐术后化疗[32]。
本研究仍然存在局限性:首先,这是一项回顾性研究,样本量较少,存在选择性偏倚以及非随机性的缺点。其次,入组患者随访过程中存在再次肝内复发以及肝外转移的患者,后续采取的不同治疗方式对生存预后可能产生不同的影响。未来仍需要多中心前瞻性随机对照试验进行验证。
综上所述,本研究结果显示,与单独MWA相比,MWA+化疗可以改善RICC的预后,延长其PFS和OS,且副反应安全可控。肿瘤直径>5 cm、复发时间<1年、未联合全身化疗的患者预后不良。
-
表 1 两组基线资料比较
Table 1. Comparison of baseline data between the two groups
项目 MWA+化疗(n=55) MWA(n=51) 统计值 P值 年龄(岁) 55.008±8.829 55.099±9.070 t=0.211 0.958 男性[例(%)] 39(70.9) 37(72.5) χ2=0.035 0.851 HBV感染 33(60.0) 27(52.9) χ2=0.537 0.464 肝硬化[例(%)] 36(65.5) 31(60.8) χ2=0.248 0.618 TBil(μmol/L) 41.025±13.796 37.208±12.988 t=1.465 0.146 AST(U/L) 65.891±23.122 73.093±24.721 t=-1.551 0.125 ALT(U/L) 69.755±24.965 79.076±35.183 t=-1.562 0.122 Alb(g/L) 38.684±4.069 38.882±3.806 t=-0.265 0.797 PT(s) 14.082±1.326 14.172±1.667 t=-0.310 0.757 INR 1.344±0.197 1.373±0.178 t=-0.806 0.421 CA19-9[例(%)] χ2=0.621 0.431 ≤37 U/mL 36(65.5) 37(72.5) >37 U/mL 19(34.5) 14(27.5) 术后复发TNM分期[例(%)] χ2=0.865 0.649 Ⅰ期 31(56.4) 26(51.0) Ⅱ期 17(30.9) 20(39.2) Ⅲ期 7(12.7) 5(9.8) 肿瘤直径[例(%)] χ2=0.907 0.119 ≤5 cm 35(63.6) 26(51.0) >5 cm 20(36.4) 25(49.0) 肿瘤个数[例(%)] χ2=0.794 0.268 1个 41(74.5) 34(66.7) 2~3个 14(25.5) 17(33.3) 首次切除时分化程度[例(%)] χ2=0.095 0.758 中高度 34(61.8) 33(64.7) 低度 21(38.2) 18(35.3) Child-Pugh分级[例(%)] χ2=0.484 0.486 A级 37(67.3) 31(60.8) B级 18(32.7) 20(39.2) 距初次切除后的复发时间[例(%)] χ2=2.358 0.125 ≤1年 33(60.0) 23(45.1) >1年 22(40.0) 28(54.9) 联合TACE[例(%)] χ2=0.028 0.867 是 38(69.1) 36(70.6) 否 17(30.9) 15(29.4) 表 2 RICC患者PFS的单因素分析
Table 2. Univariate analysis of PFS in patients with RICC
项目 HR P值 性别(男/女) 0.708(0.408~1.228) 0.219 年龄(>60/≤60岁) 1.243(0.729~2.120) 0.423 HBV(+)(是/否) 0.859(0.517~1.426) 0.557 TBil(>34.2 μmol/L/≤34.2 μmol/L) 2.498(1.497~4.169) 0.001 AST(>40 U/L/≤40 U/L) 0.754(0.343~1.657) 0.482 ALT(>40 U/L/≤40 U/L) 1.692(0.760~3.768) 0.198 Alb(>35 g/L/≤35 g/L) 1.930(1.061~3.512) 0.031 CA19-9(>37 ng/mL/≤37 ng/mL) 0.326(0.193~0.550) <0.001 TNM分期(≥Ⅱ期/Ⅰ期) 1.012(0.727~1.409) 0.943 肿瘤直径(>5 cm/≤5 cm) 0.353(0.212~0.588) <0.001 肿瘤个数(1个/2~3个) 0.778(0.453~1.335) 0.361 分化程度(中高/低) 0.472(0.287~0.775) 0.003 Child-Pugh分级(A/B) 1.165(0.690~1.969) 0.568 复发时间(>1年/≤1年) 4.286(2.505~7.331) <0.001 联合化疗(是/否) 0.437(0.255~0.747) 0.002 联合TACE(是/否) 0.756(0.446~1.281) 0.298 表 3 RICC患者OS的单因素分析
Table 3. Univariate analysis of OS in patients with RICC
项目 HR P值 性别(男/女) 0.625(0.329~1.187) 0.151 年龄(>60岁/≤60岁) 0.753(0.578~2.134) 0.753 HBV(+)(是/否) 0.754(0.413~1.376) 0.357 TBil(>34.2 μmol/L/≤34.2 μmol/L) 1.570(0.855~2.883) 0.146 AST(>40 U/L/≤40 U/L) 0.912(0.357~2.332) 0.848 ALT(>40 U/L/≤40 U/L) 2.010(0.778~5.196) 0.150 Alb(>35.0 g/L/≤35.0 g/L) 1.429(0.658~3.106) 0.367 CA19-9>40 ng/mL(是/否) 0.412(0.224~0.756) 0.004 TNM分期(≥Ⅱ期/Ⅰ期) 1.188(0.652~2.166) 0.574 肿瘤直径(>5 cm/≤5 cm) 0.244(0.127~0.470) <0.001 肿瘤个数(1个/2~3个) 0.835(0.427~1.631) 0.598 分化程度(中高/低) 0.502(0.274~0.922) 0.026 Child-Pugh分级(A/B) 0.970(0.512~1.839) 0.927 复发时间(>1年/≤1年) 2.606(1.382~4.912) 0.003 联合化疗(是/否) 0.339(0.177~0.651) 0.001 联合TACE(是/否) 0.629(0.335~1.179) 0.148 表 4 两组不良反应比较
Table 4. Comparison of complications between the two groups
项目 MWA+化疗(n=55) MWA(n=51) χ2值 P值 所有级别 3~4级 所有级别 3~4级 血液学毒性[例(%)] 18(32.7) 2(3.6) 2(3.9) 0(0.0) 12.524 <0.001 乏力[例(%)] 22(40.0) 3(5.5) 16(31.4) 1(2.0) 0.856 0.355 胃肠道反应[例(%)] 21(38.2) 2(3.6) 11(21.6) 1(2.0) 3.465 0.063 疼痛[例(%)] 18(32.7) 0(0.0) 15(29.4) 2(3.9) 0.136 0.713 肝功能异常[例(%)] 16(29.1) 1(1.8) 10(19.6) 0(0.0) 1.286 0.257 发热[例(%)] 8(14.5) 0(0.0) 4(7.8) 0(0.0) 0.277 出血[例(%)] 1(1.8) 0(0.0) 2(3.9) 0(0.0) 0.607 腹水/胸水[例(%)] 2(3.6) 0(0.0) 4(7.8) 0(0.0) 0.425 血栓[例(%)] 1(1.8) 0(0.0) 0(0.0) 0(0.0) >0.05 -
[1] KHAN SA, DAVIDSON BR, GOLDIN RD, et al. Guidelines for the diagnosis and treatment of cholangiocarcinoma: an update[J]. Gut, 2012, 61(12): 1657-1669. DOI: 10.1136/gutjnl-2011-301748. [2] DING XY, SUN W, SHEN YJ, et al. Efficacy and safety of lenvatinib combined with sintilimab as the second-line therapy for intrahepatic cholangiocarcinoma[J]. J Clin Hepatol, 2022, 38(8): 1813-1818. DOI: 10.3969/j.issn.1001-5256.2022.08.018.丁晓燕, 孙巍, 申燕军, 等. 仑伐替尼联合信迪利单抗二线治疗肝内胆管癌的效果和安全性[J]. 临床肝胆病杂志, 2022, 38(8): 1813-1818. DOI: 10.3969/j.issn.1001-5256.2022.08.018. [3] CLOYD JM, EJAZ A, PAWLIK TM. The Landmark series: Intrahepatic cholangiocarcinoma[J]. Ann Surg Oncol, 2020, 27(8): 2859-2865. DOI: 10.1245/s10434-020-08621-4. [4] DOUSSOT A, GONEN M, WIGGERS JK, et al. Recurrence patterns and disease-free survival after resection of intrahepatic cholangiocarcinoma: Preoperative and postoperative prognostic models[J]. J Am Coll Surg, 2016, 223(3): 493-505. e2. DOI: 10.1016/j.jamcollsurg.2016.05.019. [5] WRIGHT GP, PERKINS S, JONES H, et al. Surgical resection does not improve survival in multifocal intrahepatic cholangiocarcinoma: A comparison of surgical resection with intra-arterial therapies[J]. Ann Surg Oncol, 2018, 25(1): 83-90. DOI: 10.1245/s10434-017-6110-1. [6] BARTSCH F, PASCHOLD M, BAUMGART J, et al. Surgical resection for recurrent intrahepatic cholangiocarcinoma[J]. World J Surg, 2019, 43(4): 1105-1116. DOI: 10.1007/s00268-018-04876-x. [7] ZHANG XF, BEAL EW, BAGANTE F, et al. Early versus late recurrence of intrahepatic cholangiocarcinoma after resection with curative intent[J]. Br J Surg, 2018, 105(7): 848-856. DOI: 10.1002/bjs.10676. [8] LI M, LU YY, DONG JH, et al. Clinical effect of transcatheter arterial chemoembolization combined with microwave ablation in treatment of advanced primary liver cancer[J]. J Clin Hepatol, 2020, 36(12): 2720-2724. DOI: 10.3969/j.issn.1001-5256.2020.12.016.李猛, 陆荫英, 董景辉, 等. 经肝动脉化疗栓塞术联合微波消融治疗中晚期原发性肝癌的效果分析[J]. 临床肝胆病杂志, 2020, 36(12): 2720-2724. DOI: 10.3969/j.issn.1001-5256.2020.12.016. [9] HAN Y, SHAO N, XI X, et al. Use of microwave ablation in the treatment of patients with multiple primary malignant tumors[J]. Thorac Cancer, 2017, 8(4): 365-371. DOI: 10.1111/1759-7714.12445. [10] XU C, LI L, XU W, et al. Ultrasound-guided percutaneous microwave ablation versus surgical resection for recurrent intrahepatic cholangiocarcinoma: intermediate-term results[J]. Int J Hyperthermia, 2019, 36(1): 351-358. DOI: 10.1080/02656736.2019.1571247. [11] SPOLVERATO G, KIM Y, ALEXANDRESCU S, et al. Management and outcomes of patients with recurrent intrahepatic cholangiocarcinoma following previous curative-intent surgical resection[J]. Ann Surg Oncol, 2016, 23(1): 235-243. DOI: 10.1245/s10434-015-4642-9. [12] YUAN ZB, FANG HB, FENG QK, et al. Prognostic factors of recurrent intrahepatic cholangiocarcinoma after hepatectomy: A retrospective study[J]. World J Gastroenterol, 2022, 28(15): 1574-1587. DOI: 10.3748/wjg.v28.i15.1574. [13] VALLE J, WASAN H, PALMER DH, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer[J]. N Engl J Med, 2010, 362(14): 1273-1281. DOI: 10.1056/NEJMoa0908721. [14] MIURA JT, JOHNSTON FM, TSAI S, et al. Chemotherapy for surgically resected intrahepatic cholangiocarcinoma[J]. Ann Surg Oncol, 2015, 22(11): 3716-3723. DOI: 10.1245/s10434-015-4501-8. [15] BENSON AB, D'ANGELICA MI, ABBOTT DE, et al. Hepatobiliary cancers, version 2.2021, NCCN clinical practice guidelines in oncology[J]. J Natl Compr Canc Netw, 2021, 19(5): 541-565. DOI: 10.6004/jnccn.2021.0022. [16] SPOLVERATO G, EJAZ A, KIM Y, et al. Rates and patterns of recurrence after curative intent resection for gastric cancer: a United States multi-institutional analysis[J]. J Am Coll Surg, 2014, 219(4): 664-675. DOI: 10.1016/j.jamcollsurg.2014.03.062. [17] EISENHAUER EA, THERASSE P, BOGAERTS J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1)[J]. Eur J Cancer, 2009, 45(2): 228-247. DOI: 10.1016/j.ejca.2008.10.026. [18] MAVROS MN, ECONOMOPOULOS KP, ALEXIOU VG, et al. Treatment and prognosis for patients with intrahepatic cholangiocarcinoma: Systematic review and meta-analysis[J]. JAMA Surg, 2014, 149(6): 565-574. DOI: 10.1001/jamasurg.2013.5137. [19] LURJE G, BEDNARSCH J, RODERBURG C, et al. Intrahepatic cholangiocarcinoma - current perspectives and treatment algorithm[J]. Chirurg, 2018, 89(11): 858-864. DOI: 10.1007/s00104-018-0718-y. [20] TAKAHASHI Y, EBATA T, YOKOYAMA Y, et al. Surgery for recurrent biliary tract cancer: A single-center experience with 74 consecutive resections[J]. Ann Surg, 2015, 262(1): 121-129. DOI: 10.1097/SLA.0000000000000827. [21] YANG HI, SHERMAN M, SU J, et al. Nomograms for risk of hepatocellular carcinoma in patients with chronic hepatitis B virus infection[J]. J Clin Oncol, 2010, 28(14): 2437-2444. DOI: 10.1200/JCO.2009.27.4456. [22] KIM GH, KIM PH, KIM JH, et al. Thermal ablation in the treatment of intrahepatic cholangiocarcinoma: a systematic review and meta-analysis[J]. Eur Radiol, 2022, 32(2): 1205-1215. DOI: 10.1007/s00330-021-08216-x. [23] SHROFF RT, KENNEDY EB, BACHINI M, et al. Adjuvant Therapy for resected biliary tract cancer: ASCO clinical practice guideline[J]. J Clin Oncol, 2019, 37(12): 1015-1027. DOI: 10.1200/JCO.18.02178. [24] DÍAZ-GONZÁLEZÁ, VILANA R, BIANCHI L, et al. Thermal ablation for intrahepatic cholangiocarcinoma in cirrhosis: Safety and efficacy in non-surgical patients[J]. J Vasc Interv Radiol, 2020, 31(5): 710-719. DOI: 10.1016/j.jvir.2019.06.014. [25] HAN K, KO HK, KIM KW, et al. Radiofrequency ablation in the treatment of unresectable intrahepatic cholangiocarcinoma: systematic review and meta-analysis[J]. J Vasc Interv Radiol, 2015, 26(7): 943-948. DOI: 10.1016/j.jvir.2015.02.024. [26] CARRAFIELLO G, LAGANÀ D, COTTA E, et al. Radiofrequency ablation of intrahepatic cholangiocarcinoma: preliminary experience[J]. Cardiovasc Intervent Radiol, 2010, 33(4): 835-839. DOI: 10.1007/s00270-010-9849-3. [27] SHI Q, CHEN D, ZHOU C, et al. Drug-eluting beads versus lipiodol transarterial chemoembolization for the treatment of hypovascular hepatocellular carcinoma: A single-center retrospective study[J]. Cancer Manag Res, 2020, 12: 5461-5468. DOI: 10.2147/CMAR.S255960. [28] FENG R, TAO ZG, XU HY, et al. The short-term curative effect of the callisphere drug embolization microsphere in the treatment of malignant tumor of the liver[J]. J Clin Radiol, 2019, 38(6): 1107-1111. https://www.cnki.com.cn/Article/CJFDTOTAL-LCFS201906040.htm冯锐, 陶志刚, 徐后云, 等. CalliSpheres载药栓塞微球治疗肝脏乏血供恶性肿瘤短期疗效分析[J]. 临床放射学杂志, 2019, 38(6): 1107-1111. https://www.cnki.com.cn/Article/CJFDTOTAL-LCFS201906040.htm [29] CHEN X, DU J, HUANG J, et al. Neoadjuvant and adjuvant therapy in intrahepatic cholangiocarcinoma[J]. J Clin Transl Hepatol, 2022, 10(3): 553-563. DOI: 10.14218/JCTH.2021.00250. [30] STEIN A, ARNOLD D, BRIDGEWATER J, et al. Adjuvant chemotherapy with gemcitabine and cisplatin compared to observation after curative intent resection of cholangiocarcinoma and muscle invasive gallbladder carcinoma (ACTICCA-1 trial) - a randomized, multidisciplinary, multinational phase Ⅲ trial[J]. BMC Cancer, 2015, 15: 564. DOI: 10.1186/s12885-015-1498-0. [31] EDELINE J, BENABDELGHANI M, BERTAUT A, et al. Gemcitabine and oxaliplatin chemotherapy or surveillance in resected biliary tract cancer (PRODIGE 12-ACCORD 18-UNICANCER GI): A Randomized Phase Ⅲ Study[J]. J Clin Oncol, 2019, 37(8): 658-667. DOI: 10.1200/JCO.18.00050. [32] MAZZAFERRO V, GORGEN A, ROAYAIE S, et al. Liver resection and transplantation for intrahepatic cholangiocarcinoma[J]. J Hepatol, 2020, 72(2): 364-377. DOI: 10.1016/j.jhep.2019.11.020. -




 PDF下载 ( 2115 KB)
PDF下载 ( 2115 KB)

 下载:
下载:



 下载:
下载: