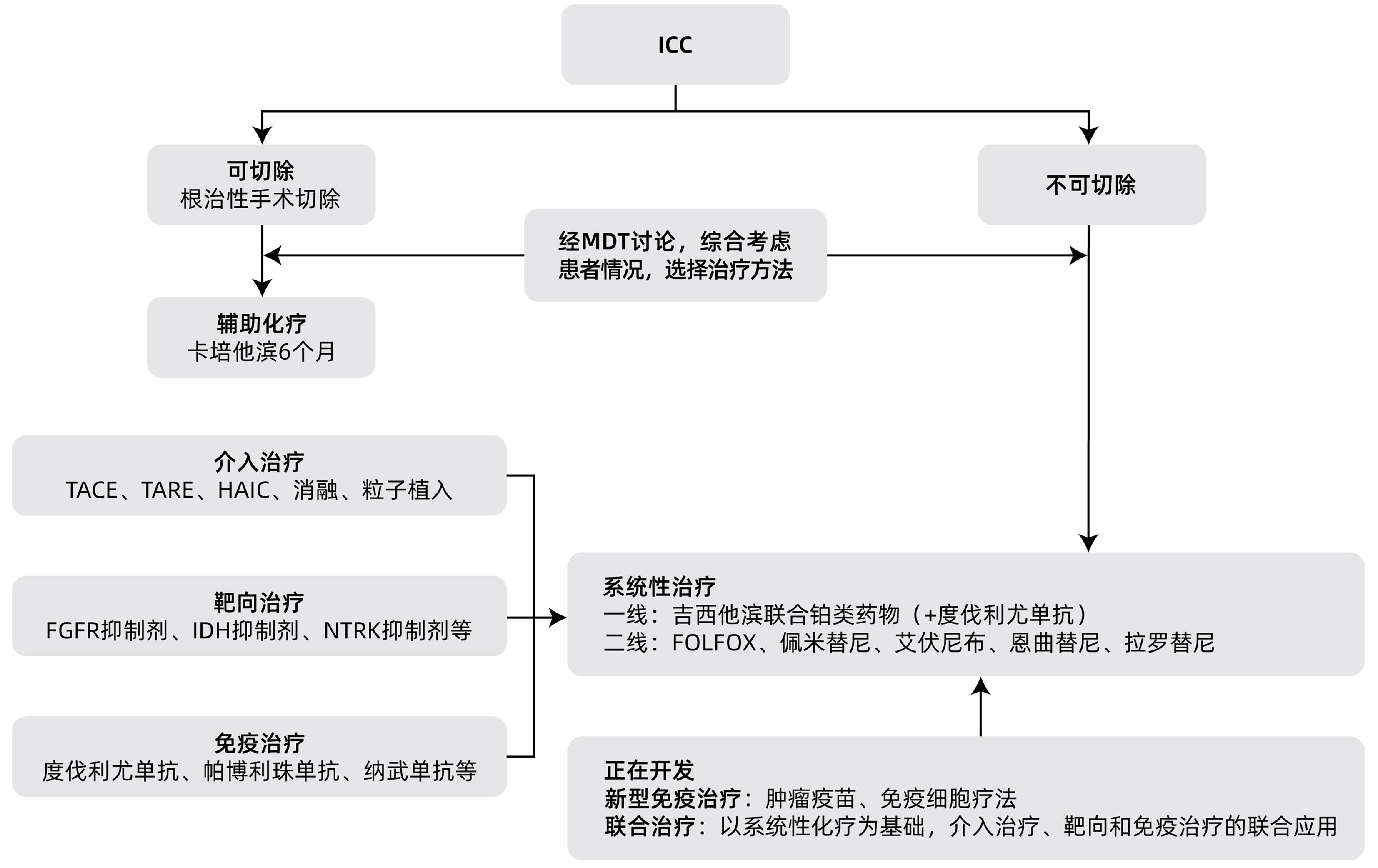
| [1] |
SIEGEL RL, MILLER KD, FUCHS HE, et al. Cancer statistics, 2022[J]. CA Cancer J Clin, 2022, 72( 1): 7- 33. DOI: 10.3322/caac.21708. |
| [2] |
DASGUPTA P, HENSHAW C, YOULDEN DR, et al. Global trends in incidence rates of primary adult liver cancers: A systematic review and meta-analysis[J]. Front Oncol, 2020, 10: 171. DOI: 10.3389/fonc.2020.00171. |
| [3] |
BANALES JM, MARIN JJG, LAMARCA A, et al. Cholangiocarcinoma 2020: The next horizon in mechanisms and management[J]. Nat Rev Gastroenterol Hepatol, 2020, 17( 9): 557- 588. DOI: 10.1038/s41575-020-0310-z. |
| [4] |
SUNG H, FERLAY J, SIEGEL RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA Cancer J Clin, 2021, 71( 3): 209- 249. DOI: 10.3322/caac.21660. |
| [5] |
CHEN CB, NELSON LJ, ÁVILA MA, et al. Mitogen-activated protein kinases(MAPKs) and cholangiocarcinoma: The missing link[J]. Cells, 2019, 8( 10): 1172. DOI: 10.3390/cells8101172. |
| [6] |
LEE AJ, CHUN YS. Intrahepatic cholangiocarcinoma: The AJCC/UICC 8th edition updates[J]. Chin Clin Oncol, 2018, 7( 5): 52. DOI: 10.21037/cco.2018.07.03. |
| [7] |
MALAGUARNERA G, PALADINA I, GIORDANO M, et al. Serum markers of intrahepatic cholangiocarcinoma[J]. Dis Markers, 2013, 34( 4): 219- 228. DOI: 10.3233/DMA-130964. |
| [8] |
MORO A, MEHTA R, SAHARA K, et al. The impact of preoperative CA19-9 and CEA on outcomes of patients with intrahepatic cholangiocarcinoma[J]. Ann Surg Oncol, 2020, 27( 8): 2888- 2901. DOI: 10.1245/s10434-020-08350-8. |
| [9] |
OHAEGBULAM KC, KOETHE Y, FUNG A, et al. The multidisciplinary management of cholangiocarcinoma[J]. Cancer, 2023, 129( 2): 184- 214. DOI: 10.1002/cncr.34541. |
| [10] |
LAPITZ A, AZKARGORTA M, MILKIEWICZ P, et al. Liquid biopsy-based protein biomarkers for risk prediction, early diagnosis, and prognostication of cholangiocarcinoma[J]. J Hepatol, 2023, 79( 1): 93- 108. DOI: 10.1016/j.jhep.2023.02.027. |
| [11] |
KIM DW, KIM SY, YOO C, et al. Update on biliary cancer imaging[J]. Radiol Clin North Am, 2022, 60( 5): 825- 842. DOI: 10.1016/j.rcl.2022.05.001. |
| [12] |
FÁBREGA-FOSTER K, GHASABEH MA, PAWLIK TM, et al. Multimodality imaging of intrahepatic cholangiocarcinoma[J]. Hepatobiliary Surg Nutr, 2017, 6( 2): 67- 78. DOI: 10.21037/hbsn.2016.12.10. |
| [13] |
Expert Committee of Expert Consensus on Pathological Diagnosis of Intrahepatic Cholangiocarcinoma( 2022 version). Expert consensus on pathological diagnosis of intrahepatic cholangiocarcinoma(2022 version)[J]. Chin J Pathol, 2022, 51( 9): 819- 827. DOI: 10.3760/cma.j.cn112151-20220517-00423. |
| [14] |
SIA D, HOSHIDA Y, VILLANUEVA A, et al. Integrative molecular analysis of intrahepatic cholangiocarcinoma reveals 2 classes that have different outcomes[J]. Gastroenterology, 2013, 144( 4): 829- 840. DOI: 10.1053/j.gastro.2013.01.001. |
| [15] |
KOMUTA M. Intrahepatic cholangiocarcinoma: Tumour heterogeneity and its clinical relevance[J]. Clin Mol Hepatol, 2022, 28( 3): 396- 407. DOI: 10.3350/cmh.2021.0287. |
| [16] |
DONG LQ, LU DY, CHEN R, et al. Proteogenomic characterization identifies clinically relevant subgroups of intrahepatic cholangiocarcinoma[J]. Cancer Cell, 2022, 40( 1): 70- 87. DOI: 10.1016/j.ccell.2021.12.006. |
| [17] |
CHUN YS, JAVLE M. Systemic and adjuvant therapies for intrahepatic cholangiocarcinoma[J]. Cancer Control, 2017, 24( 3): 1073274817729241. DOI: 10.1177/1073274817729241. |
| [18] |
NIU YJ, ZHA Y, LI SJ, et al. Analysis on prognosis related factors of patients with cholangiocarcinoma after radical resection and establishment of survival prediction model[J]. J Jilin Univ(Med Edit), 2022, 48( 4): 979- 987. DOI: 10.13481/j.1671-587X.20220418. |
| [19] |
PRIMROSE JN, FOX RP, PALMER DH, et al. Capecitabine compared with observation in resected biliary tract cancer(BILCAP): A randomised, controlled, multicentre, phase 3 study[J]. Lancet Oncol, 2019, 20( 5): 663- 673. DOI: 10.1016/S1470-2045(18)30915-X. |
| [20] |
Chinese Society of Liver Cancer Cholangiocarcinoma Cooperative Group. Chinese expert consensus on management of intrahepatic cholangiocarcinoma(2022 edition)[J]. Chin J Dig Surg, 2022, 21( 10): 1269- 1301. DOI: 10.3760/cma.j.cn115610-20220829-00476. |
| [21] |
BOWLUS CL, ARRIVÉ L, BERGQUIST A, et al. AASLD practice guidance on primary sclerosing cholangitis and cholangiocarcinoma[J]. Hepatology, 2023, 77( 2): 659- 702. DOI: 10.1002/hep.32771. |
| [22] |
European Association for the Study of the Liver. EASL-ILCA Clinical Practice Guidelines on the management of intrahepatic cholangiocarcinoma[J]. J Hepatol, 2023, 79( 1): 181- 208. DOI: 10.1016/j.jhep.2023.03.010. |
| [23] |
BUETTNER S, van VUGT JL, IJZERMANS JN, et al. Intrahepatic cholangiocarcinoma: Current perspectives[J]. Onco Targets Ther, 2017, 10: 1131- 1142. DOI: 10.2147/OTT.S93629. |
| [24] |
BUETTNER S, KOERKAMP BG, EJAZ A, et al. The effect of preoperative chemotherapy treatment in surgically treated intrahepatic cholangiocarcinoma patients-a multi-institutional analysis[J]. J Surg Oncol, 2017, 115( 3): 312- 318. DOI: 10.1002/jso.24524. |
| [25] |
FANG TL, XIAO JY, ZHANG YR, et al. Combined with interventional therapy, immunotherapy can create a new outlook for tumor treatment[J]. Quant Imaging Med Surg, 2021, 11( 6): 2837- 2860. DOI: 10.21037/qims-20-173. |
| [26] |
MARTIN RCG 2, SIMO KA, HANSEN P, et al. Drug-eluting bead, irinotecan therapy of unresectable intrahepatic cholangiocarcinoma(DELTIC) with concomitant systemic gemcitabine and cisplatin[J]. Ann Surg Oncol, 2022, 29( 9): 5462- 5473. DOI: 10.1245/s10434-022-11932-3. |
| [27] |
EDELINE J, TOUCHEFEU Y, GUIU B, et al. Radioembolization plus chemotherapy for first-line treatment of locally advanced intrahepatic cholangiocarcinoma: A phase 2 clinical trial[J]. JAMA Oncol, 2020, 6( 1): 51- 59. DOI: 10.1001/jamaoncol.2019.3702. |
| [28] |
CERCEK A, BOERNER T, TAN BR, et al. Assessment of hepatic arterial infusion of floxuridine in combination with systemic gemcitabine and oxaliplatin in patients with unresectable intrahepatic cholangiocarcinoma: A phase 2 clinical trial[J]. JAMA Oncol, 2020, 6( 1): 60- 67. DOI: 10.1001/jamaoncol.2019.3718. |
| [29] |
MEI J, TANG YH, WEI W, et al. Hepatic arterial infusion chemotherapy combined with PD-1 inhibitors plus lenvatinib versus PD-1 inhibitors plus lenvatinib for advanced hepatocellular carcinoma[J]. Front Oncol, 2021, 11: 618206. DOI: 10.3389/fonc.2021.618206. |
| [30] |
ZHANG QY, LIU XY, WEI SM, et al. Lenvatinib plus PD-1 inhibitors as first-line treatment in patients with unresectable biliary tract cancer: A single-arm, open-label, phase II study[J]. Front Oncol, 2021, 11: 751391. DOI: 10.3389/fonc.2021.751391. |
| [31] |
VALLE J, WASAN H, PALMER DH, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer[J]. N Engl J Med, 2010, 362( 14): 1273- 1281. DOI: 10.1056/NEJMoa0908721. |
| [32] |
MORIZANE C, OKUSAKA T, MIZUSAWA J, et al. Combination gemcitabine plus S-1 versus gemcitabine plus cisplatin for advanced/recurrent biliary tract cancer: The FUGA-BT(JCOG1113) randomized phase III clinical trial[J]. Ann Oncol, 2019, 30( 12): 1950- 1958. DOI: 10.1093/annonc/mdz402. |
| [33] |
KIM ST, KANG JH, LEE J, et al. Capecitabine plus oxaliplatin versus gemcitabine plus oxaliplatin as first-line therapy for advanced biliary tract cancers: A multicenter, open-label, randomized, phase III, noninferiority trial[J]. Ann Oncol, 2019, 30( 5): 788- 795. DOI: 10.1093/annonc/mdz058. |
| [34] |
SHROFF RT, JAVLE MM, XIAO LC, et al. Gemcitabine, cisplatin, and nab-paclitaxel for the treatment of advanced biliary tract cancers: A phase 2 clinical trial[J]. JAMA Oncol, 2019, 5( 6): 824- 830. DOI: 10.1001/jamaoncol.2019.0270. |
| [35] |
IOKA T, KANAI M, KOBAYASHI S, et al. Randomized phase III study of gemcitabine, cisplatin plus S-1 versus gemcitabine, cisplatin for advanced biliary tract cancer(KHBO1401- MITSUBA)[J]. J Hepatobiliary Pancreat Sci, 2023, 30( 1): 102- 110. DOI: 10.1002/jhbp.1219. |
| [36] |
LAMARCA A, PALMER DH, WASAN HS, et al. Second-line FOLFOX chemotherapy versus active symptom control for advanced biliary tract cancer(ABC-06): A phase 3, open-label, randomised, controlled trial[J]. Lancet Oncol, 2021, 22( 5): 690- 701. DOI: 10.1016/S1470-2045(21)00027-9. |
| [37] |
|
| [38] |
ABOU-ALFA G, SAHAI V, HOLLEBECQUE A, et al. Pemigatinib for previously treated locally advanced/metastatic cholangiocarcinoma(CCA): Update of FIGHT-202[J]. J Clin Oncol, 2021, 39: 4086. DOI: 10.1200/JCO.2021.39.15_SUPPL.4086. |
| [39] |
SHI GM, HUANG XY, WEN TF, et al. Pemigatinib in Chinese patients with advanced/metastatic or surgically unresectable cholangiocarcinoma including FGFR2 fusion or rearrangement: Updated data from an open-label, single-arm, multicenter phase II study(CIBI375A201 study)[J]. J Clin Oncol, 2022, 40( 16_suppl): e16183. DOI: 10.1200/jco.2022.40.16_suppl.e16183. |
| [40] |
RIZZO A, RICCI AD, BRANDI G. IDH inhibitors in advanced cholangiocarcinoma: Another arrow in the quiver?[J]. Cancer Treat Res Commun, 2021, 27: 100356. DOI: 10.1016/j.ctarc.2021.100356. |
| [41] |
ABOU-ALFA GK, MACARULLA T, JAVLE MM, et al. Ivosidenib in IDH1-mutant, chemotherapy-refractory cholangiocarcinoma(ClarIDHy): A multicentre, randomised, double-blind, placebo-controlled, phase 3 study[J]. Lancet Oncol, 2020, 21( 6): 796- 807. DOI: 10.1016/S1470-2045(20)30157-1. |
| [42] |
DU JJ, LV X, ZHANG ZY, et al. Revisiting targeted therapy and immunotherapy for advanced cholangiocarcinoma[J]. Front Immunol, 2023, 14: 1142690. DOI: 10.3389/fimmu.2023.1142690. |
| [43] |
DOEBELE RC, DRILON A, PAZ-ARES L, et al. Entrectinib in patients with advanced or metastatic NTRK fusion-positive solid tumours: Integrated analysis of three phase 1-2 trials[J]. Lancet Oncol, 2020, 21( 2): 271- 282. DOI: 10.1016/S1470-2045(19)30691-6. |
| [44] |
DRILON A, LAETSCH TW, KUMMAR S, et al. Efficacy of larotrectinib in TRK fusion-positive cancers in adults and children[J]. N Engl J Med, 2018, 378( 8): 731- 739. DOI: 10.1056/NEJMoa1714448. |
| [45] |
VALLE JW, LAMARCA A, GOYAL L, et al. New horizons for precision medicine in biliary tract cancers[J]. Cancer Discov, 2017, 7( 9): 943- 962. DOI: 10.1158/2159-8290.CD-17-0245. |
| [46] |
OHBA A, MORIZANE C, UENO M, et al. Multicenter phase II trial of trastuzumab deruxtecan for HER2-positive unresectable or recurrent biliary tract cancer: HERB trial[J]. Future Oncol, 2022, 18( 19): 2351- 2360. DOI: 10.2217/fon-2022-0214. |
| [47] |
|
| [48] |
BERI N. Immune checkpoint inhibitors in cholangiocarcinoma[J]. Immunotherapy, 2023, 15( 7): 541- 551. DOI: 10.2217/imt-2022-0288. |
| [49] |
MARABELLE A, LE DT, ASCIERTO PA, et al. Efficacy of pembrolizumab in patients with noncolorectal high microsatellite instability/mismatch repair-deficient cancer: Results from the phase II KEYNOTE-158 study[J]. J Clin Oncol, 2020, 38( 1): 1- 10. DOI: 10.1200/JCO.19.02105. |
| [50] |
BENSON AB, D’ANGELICA MI, ABBOTT DE, et al. Hepatobiliary cancers, version 2.2021, NCCN clinical practice guidelines in oncology[J]. J Natl Compr Canc Netw, 2021, 19( 5): 541- 565. DOI: 10.6004/jnccn.2021.0022. |
| [51] |
OVERMAN MJ, LONARDI S, WONG KYM, et al. Durable clinical benefit with nivolumab plus ipilimumab in DNA mismatch repair-deficient/microsatellite instability-high metastatic colorectal cancer[J]. J Clin Oncol, 2018, 36( 8): 773- 779. DOI: 10.1200/JCO.2017.76.9901. |
| [52] |
HODI FS, CHIARION-SILENI V, GONZALEZ R, et al. Nivolumab plus ipilimumab or nivolumab alone versus ipilimumab alone in advanced melanoma(CheckMate 067): 4-year outcomes of a multicentre, randomised, phase 3 trial[J]. Lancet Oncol, 2018, 19( 11): 1480- 1492. DOI: 10.1016/S1470-2045(18)30700-9. |
| [53] |
IOKA T, UENO M, OH DY, et al. Evaluation of safety and tolerability of durvalumab(D) with or without tremelimumab(T) in patients(pts) with biliary tract cancer(BTC)[J]. J Clin Oncol, 2019, 37( 4_suppl): 387. DOI: 10.1200/jco.2019.37.4_suppl.387. |
| [54] |
VILLANUEVA L, LWIN Z, CHUNG HC, et al. Lenvatinib plus pembrolizumab for patients with previously treated biliary tract cancers in the multicohort phase II LEAP-005 study[J]. J Clin Oncol, 2021, 39( 3_suppl): 321. DOI: 10.1200/jco.2021.39.3_suppl.321. |
| [55] |
BURRIS HA 3, OKUSAKA T, VOGEL A, et al. Durvalumab plus gemcitabine and cisplatin in advanced biliary tract cancer(TOPAZ-1): Patient-reported outcomes from a randomised, double-blind, placebo-controlled, phase 3 trial[J]. Lancet Oncol, 2024, 25( 5): 626- 635. DOI: 10.1016/S1470-2045(24)00082-2. |
| [56] |
KELLEY RK, UENO M, YOO C, et al. Pembrolizumab in combination with gemcitabine and cisplatin compared with gemcitabine and cisplatin alone for patients with advanced biliary tract cancer(KEYNOTE-966): A randomised, double-blind, placebo-controlled, phase 3 trial[J]. Lancet, 2023, 401( 10391): 1853- 1865. DOI: 10.1016/S0140-6736(23)00727-4. |
| [57] |
JIAN Z, FAN J, SHI GM, et al. Gemox chemotherapy in combination with anti-PD1 antibody toripalimab and lenvatinib as first-line treatment for advanced intrahepatic cholangiocarcinoma: A phase 2 clinical trial[J]. J Clin Oncol, 2021, 39( 15_suppl): 4094. DOI: 10.1200/jco.2021.39.15_suppl.4094. |
| [58] |
WANG K, LIU ZH, YU HM, et al. Efficacy and safety of a triple combination of atezolizumab, bevacizumab plus GEMOX for advanced biliary tract cancer: A multicenter, single-arm, retrospective study[J]. Therap Adv Gastroenterol, 2023, 16: 17562848231160630. DOI: 10.1177/17562848231160630. |
| [59] |
SHIRAHAMA T, MUROYA D, MATSUEDA S, et al. A randomized phase II trial of personalized peptide vaccine with low dose cyclophosphamide in biliary tract cancer[J]. Cancer Sci, 2017, 108( 5): 838- 845. DOI: 10.1111/cas.13193. |
| [60] |
GOLDSTEIN D, LEMECH C, VALLE J. New molecular and immunotherapeutic approaches in biliary cancer[J]. ESMO Open, 2017, 2( Suppl 1): e000152. DOI: 10.1136/esmoopen-2016-000152. |
| [61] |
FENG KC, LIU Y, GUO YL, et al. Phase I study of chimeric antigen receptor modified T cells in treating HER2-positive advanced biliary tract cancers and pancreatic cancers[J]. Protein Cell, 2018, 9( 10): 838- 847. DOI: 10.1007/s13238-017-0440-4. |
| [62] |
TRAN E, TURCOTTE S, GROS A, et al. Cancer immunotherapy based on mutation-specific CD4 + T cells in a patient with epithelial cancer[J]. Science, 2014, 344( 6184): 641- 645. DOI: 10.1126/science.1251102. |



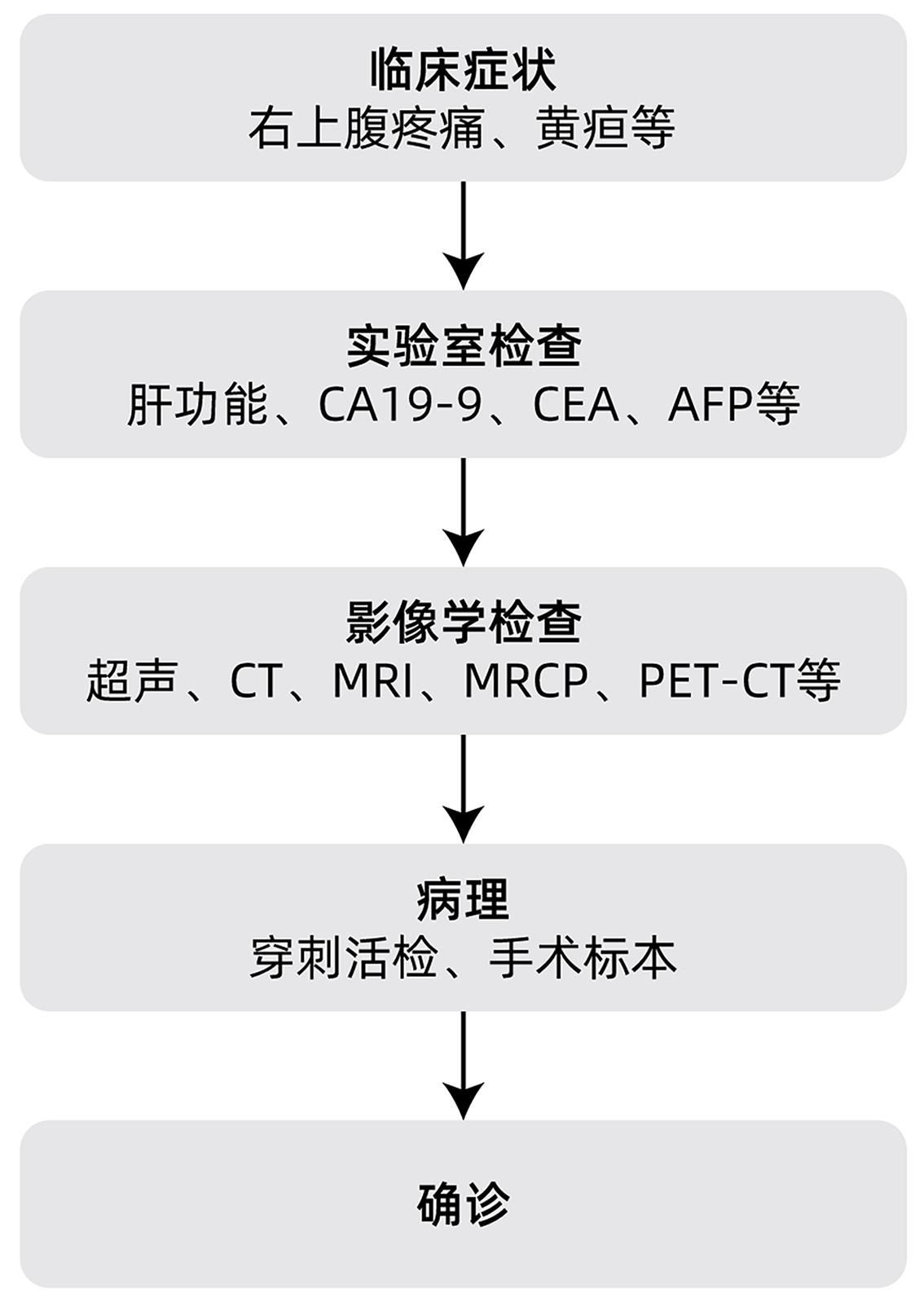




 DownLoad:
DownLoad: