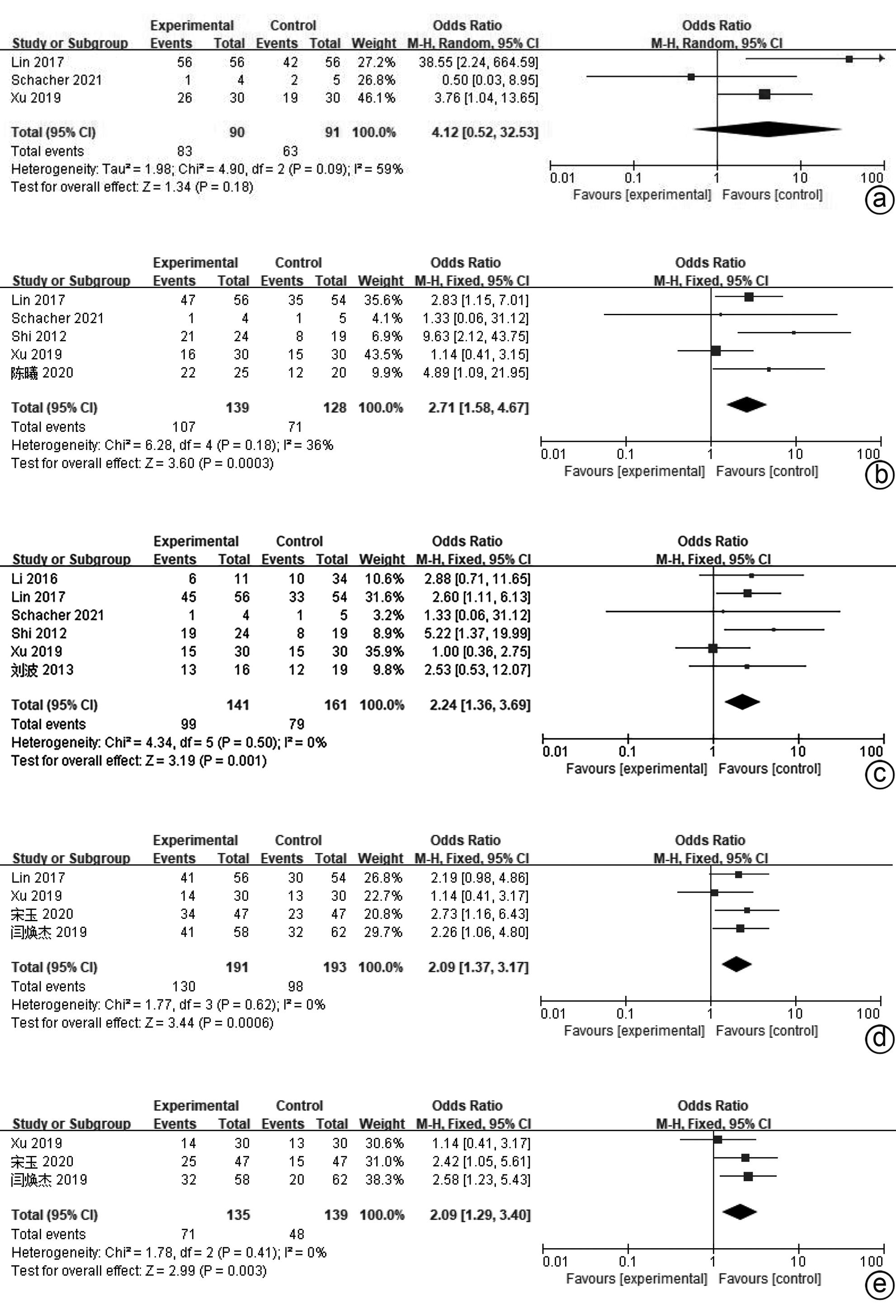
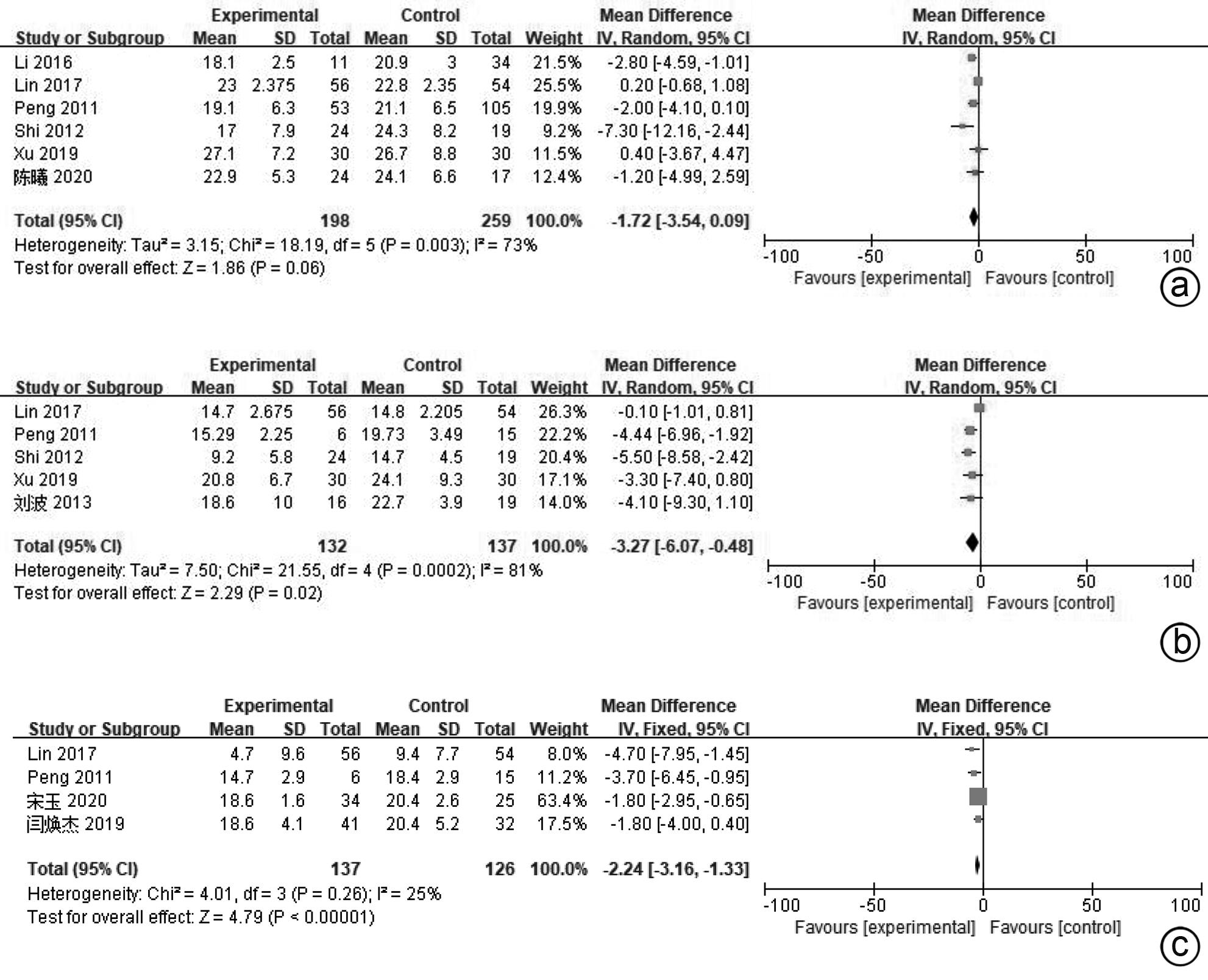
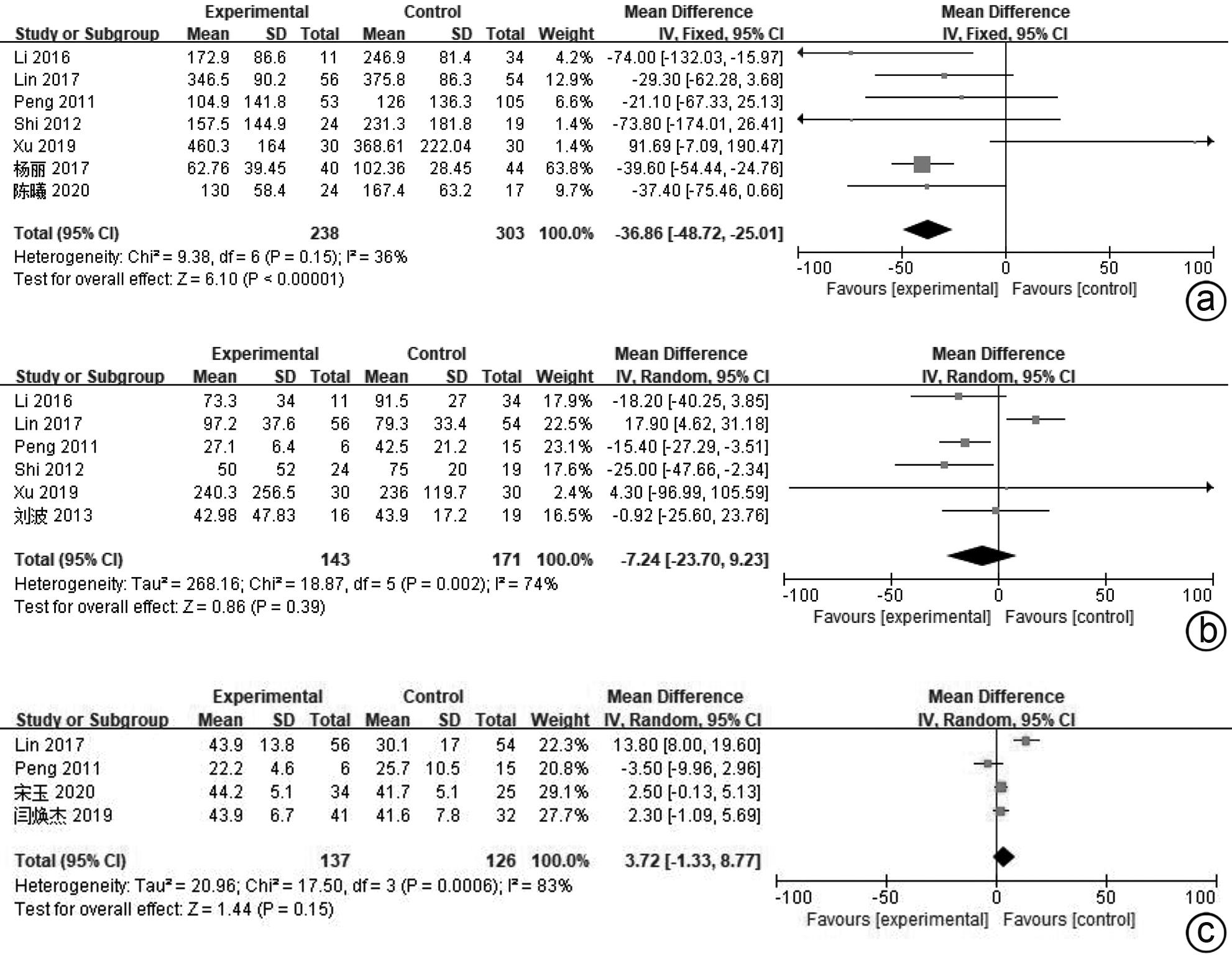
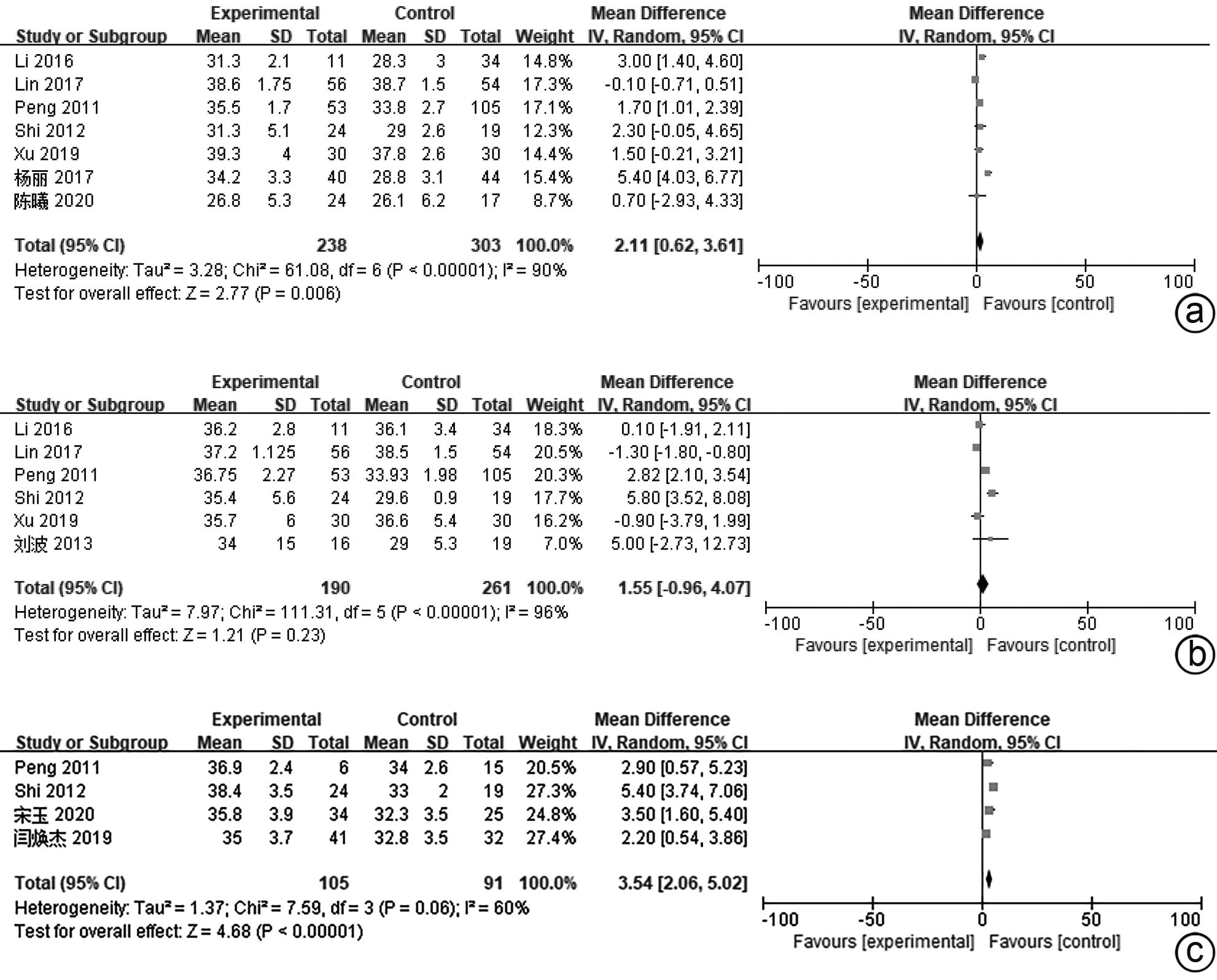
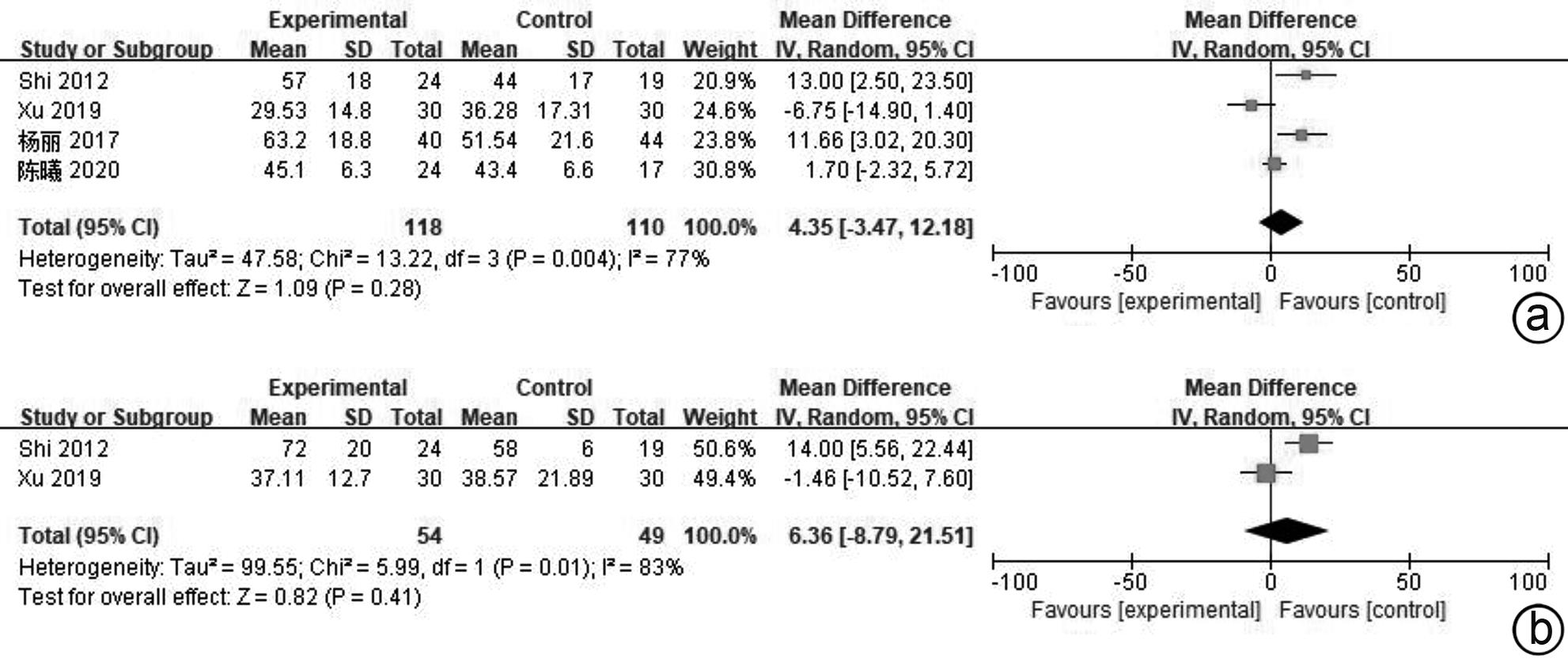
| [1] |
SARIN SK, CHOUDHURY A, SHARMA MK, et al. Acute-on-chronic liver failure: Consensus recommendations of the Asian Pacific association for the study of the liver(APASL): An update[J]. Hepatol Int, 2019, 13( 4): 353- 390. DOI: 10.1007/s12072-019-09946-3. |
| [2] |
Group of Stem Cell Engineering, Medical Engineering Society of Chinese Medical Association. Expert consensus on standardized treatment of decompensated liver cirrhosis with stem cell transplantation(2021)[J]. J Clin Hepatol, 2021, 37( 7): 1540- 1544. DOI: 10.3969/j.issn.1001-5256.2021.07.012. |
| [3] |
LIU JX, GAO JF, LIANG ZX, et al. Mesenchymal stem cells and their microenvironment[J]. Stem Cell Res Ther, 2022, 13( 1): 429. DOI: 10.1186/s13287-022-02985-y. |
| [4] |
LI YH, XU Y, WU HM, et al. Umbilical cord-derived mesenchymal stem cell transplantation in hepatitis B virus related acute-on-chronic liver failure treated with plasma exchange and entecavir: A 24-month prospective study[J]. Stem Cell Rev Rep, 2016, 12( 6): 645- 653. DOI: 10.1007/s12015-016-9683-3. |
| [5] |
LIN BL, CHEN JF, QIU WH, et al. Allogeneic bone marrow-derived mesenchymal stromal cells for hepatitis B virus-related acute-on-chronic liver failure: A randomized controlled trial[J]. Hepatology, 2017, 66( 1): 209- 219. DOI: 10.1002/hep.29189. |
| [6] |
SCHACHER FC, MARTINS PEZZI DA SILVA A, SILLA LMDR, et al. Bone marrow mesenchymal stem cells in acute-on-chronic liver failure grades 2 and 3: A phase I-II randomized clinical trial[J]. Can J Gastroenterol Hepatol, 2021, 2021: 3662776. DOI: 10.1155/2021/3662776. |
| [7] |
SHI M, ZHANG Z, XU RN, et al. Human mesenchymal stem cell transfusion is safe and improves liver function in acute-on-chronic liver failure patients[J]. Stem Cells Transl Med, 2012, 1( 10): 725- 731. DOI: 10.5966/sctm.2012-0034. |
| [8] |
XU WX, HE HL, PAN SW, et al. Combination treatments of plasma exchange and umbilical cord-derived mesenchymal stem cell transplantation for patients with hepatitis B virus-related acute-on-chronic liver failure: A clinical trial in China[J]. Stem Cells Int, 2019, 2019: 4130757. DOI: 10.1155/2019/4130757. |
| [9] |
CHEN X, CHEN ZL, DONG J, et al. Efficacy of human umbilical cord-derived mesenchymal stem cell transplantation at base of double plasma molecular absorption system in treamtment of patients with acute-on-chronic liver failure[J]. J Pract Hepatol, 2020, 23( 4): 544- 547. DOI: 10.3969/j.issn.1672-5069.2020.04.023. |
| [10] |
LIU B, DONG J, ZHANG JF, et al. Efficacy of human umbilical cord-derived mesenchymal stem cells in treatment of patients with subacute-on-chronic liver failure[J]. J Pract Hepatol, 2013, 16( 1): 29- 31. DOI: 10.3969/j.issn.1672-5069.2013.01.010. |
| [11] |
SONG Y, WANG F. Efficacy of transhepatic artery autologous bone marrow stem cell transplantation in the treatment of patients with hepatitis B-induced acute-on-chronic liver failure[J]. J Pract Hepatol, 2020, 23( 3): 388- 391. DOI: 10.3969/j.issn.1672-5069.2020.03.022. |
| [12] |
YAN HJ, ZHANG SQ, ZHAO WJ. Therapeutic efficacy of autologous bone marrow stem cell transplantation for treatment of patients with hepatitis B-induced acute-on-chronic liver failure[J]. J Pract Hepatol, 2019, 22( 1): 81- 84. DOI: 10.3969/j.issn.1672-5069.2019.01.022. |
| [13] |
YANG L, MA YJ, LIU GJ, et al. Study of human umbilical cord derived mesenchymal stem cells in treatment of HBV-related acute-on-chronic liver failure[J]. J Med Res, 2017, 46( 6): 151- 153. DOI: 10.11969/j.issn.1673-548X.2017.06.039. |
| [14] |
PENG L, XIE DY, LIN BL, et al. Autologous bone marrow mesenchymal stem cell transplantation in liver failure patients caused by hepatitis B: short-term and long-term outcomes[J]. Hepatology, 2011, 54( 3): 820- 828. DOI: 10.1002/hep.24434. |
| [15] |
|
| [16] |
FORBES SJ, GUPTA S, DHAWAN A. Cell therapy for liver disease: From liver transplantation to cell factory[J]. J Hepatol, 2015, 62( 1 Suppl): S157- S169. DOI: 10.1016/j.jhep.2015.02.040. |
| [17] |
ZHANG B, DILIHUMAER ZYE, ZHANG SY, et al. Progress on pathogenesis and medical treatment of hepatitis B virus-related chronic and acute liver failure[J/CD]. Chin J Liver Dis(Electronic Version), 2023, 15( 1): 28- 33. DOI: 10.3969/j.issn.1674-7380.2023.01.005. |
| [18] |
DONG JL, CHEN Y. Prognostic grade of acute-on-chronic liver failure: Different outcomes of the same disease[J]. J Clin Hepatol, 2021, 37( 9): 2030- 2032. DOI: 10.3969/j.issn.1001-5256.2021.09.006. |
| [19] |
WONG F, PIANO S, SINGH V, et al. Clinical features and evolution of bacterial infection-related acute-on-chronic liver failure[J]. J Hepatol, 2021, 74( 2): 330- 339. DOI: 10.1016/j.jhep.2020.07.046. |
| [20] |
SHI M, LI YY, XU RN, et al. Mesenchymal stem cell therapy in decompensated liver cirrhosis: A long-term follow-up analysis of the randomized controlled clinical trial[J]. Hepatol Int, 2021, 15( 6): 1431- 1441. DOI: 10.1007/s12072-021-10199-2. |
| [21] |
WENG WZ, CHEN JF, MEI YY, et al. Treatment effect of allogeneic bone marrow mesenchymal stem cells transplantation to patients in different phase of HBV acute-on-chronic liver failure[J]. J Sun Yat-Sen Univ(Med Sci), 2013, 34( 3): 422- 428. DOI: 10.13471/j.cnki.j.sun.yat-sen.univ(med.sci).2013.0067. |
| [22] |
ZHOU GP, JIANG YZ, SUN LY, et al. Therapeutic effect and safety of stem cell therapy for chronic liver disease: A systematic review and meta-analysis of randomized controlled trials[J]. Stem Cell Res Ther, 2020, 11( 1): 419. DOI: 10.1186/s13287-020-01935-w. |
| [23] |
LALU MM, MCINTYRE L, PUGLIESE C, et al. Safety of cell therapy with mesenchymal stromal cells(SafeCell): A systematic review and meta-analysis of clinical trials[J]. PLoS One, 2012, 7( 10): e47559. DOI: 10.1371/journal.pone.0047559. |
| [24] |
CAPLAN AI. Mesenchymal stem cells: Time to change the name![J]. Stem Cells Transl Med, 2017, 6( 6): 1445- 1451. DOI: 10.1002/sctm.17-0051. |
| [25] |
LI JJ, XUN YH. Research advances on the immunomodulatory role of mesenchymal stem cells and their exosomes in acute liver failure[J]. Zhejiang Med J, 2023, 45( 21): 2338- 2343. DOI: 10.12056/j.issn.1006-2785.2023.45.21.2023-731. |
| [26] |
WANG ZR, ZHU B, YU LM, et al. Role of stem cell-derived exosomes in treatment of liver diseases[J]. J Clin Hepatol, 2023, 39( 3): 699- 706. DOI: 10.3969/j.issn.1001-5256.2023.03.034. |



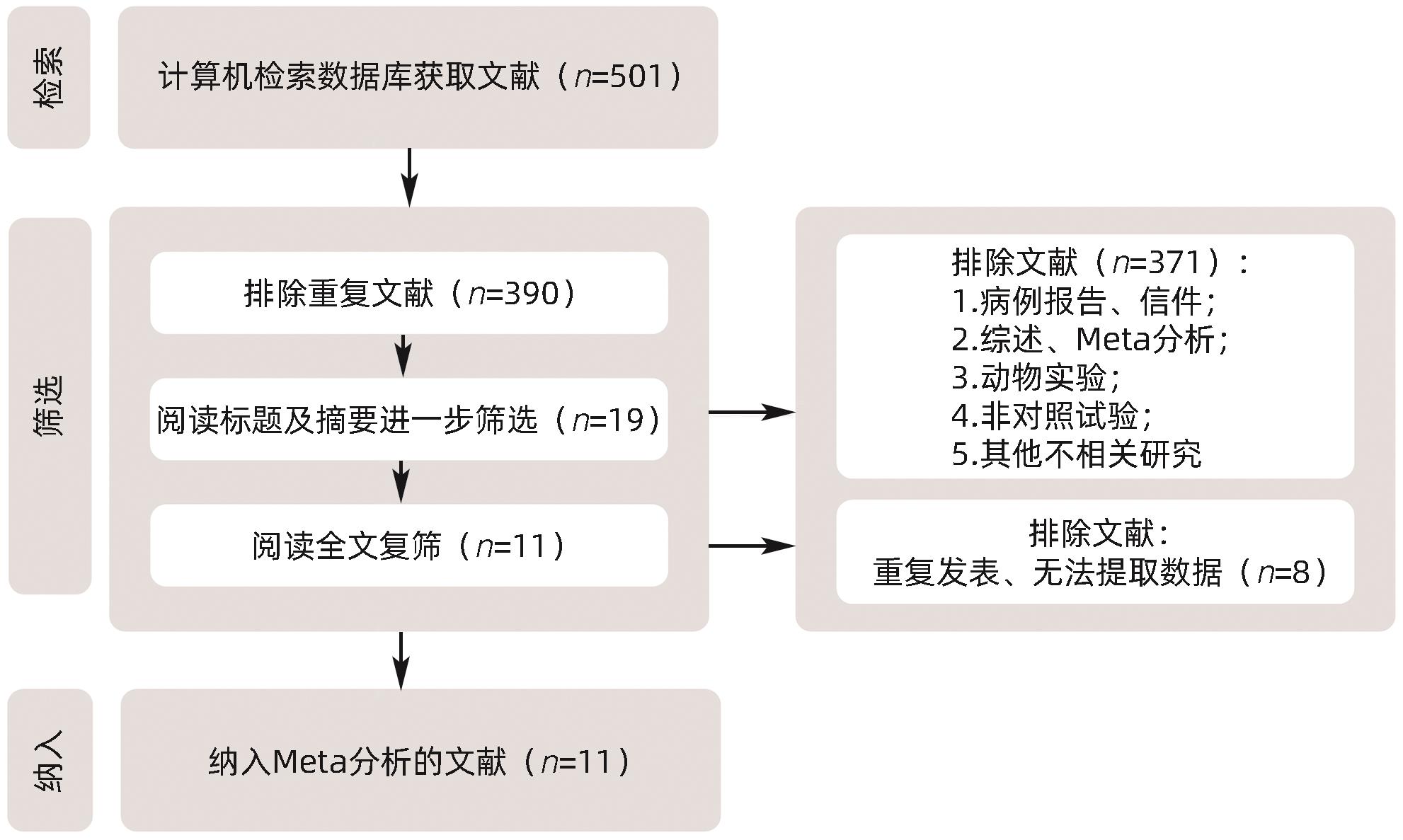




 DownLoad:
DownLoad: