| [1] |
LI X, ZHANG L, PU CM, et al. Liver transplantation in acute-on-chronic liver failure: Timing of transplantation and selection of patient population[J]. Front Med(Lausanne), 2022, 9: 1030336. DOI: 10.3389/fmed.2022.1030336. |
| [2] |
Hepatology Branch of Chinese Medical Association, Infectious Diseases Branch of Chinese Medical Association. Guidelines for the prevention and treatment of chronic hepatitis B(version 2022)[J]. Chin J Infect Dis, 2023, 41( 1): 3- 28. DOI: 10.3760/cma.j.cn311365-20230220-00050. |
| [3] |
LIU L, HAN T, CAI JJ, et al. Clinical characteristics and progression risk factors for hepatitis B-related acute-on-chronic liver failure in elderly patients[J]. Chin J Geriatr, 2022, 41( 1): 51- 56. DOI: 10.3760/cma.j.issn.0254-9026.2022.01.011. |
| [4] |
WANG X, ZHAO M, ZHANG C, et al. Establishment and clinical application of the nomogram related to risk or prognosis of hepatocellular carcinoma: A review[J]. J Hepatocell Carcinoma, 2023, 10: 1389- 1398. DOI: 10.2147/jhc.s417123. |
| [5] |
ZHANG LJ, TANG L, CHEN SY, et al. A nomogram for predicting the 4-year risk of chronic kidney disease among Chinese elderly adults[J]. Int Urol Nephrol, 2023, 55( 6): 1609- 1617. DOI: 10.1007/s11255-023-03470-y. |
| [6] |
CAI ZM, LIN HM, LI ZX, et al. A prediction nomogram for postoperative gastroparesis syndrome in right colon cancer: A retrospective study[J]. Langenbecks Arch Surg, 2023, 408( 1): 148. DOI: 10.1007/s00423-023-02885-6. |
| [7] |
Liver Failure and Artificial Liver Group, Chinese Society of Infectious Diseases, Chinese Medical Association; Severe Liver Disease and Artificial Liver Group, Chinese Society of Hepatology, Chinese Medical Association. Guideline for diagnosis and treatment of liver failure(2018)[J]. J Clin Hepatol, 2019, 35( 1): 38- 44. DOI: 10.3969/j.issn.1001-5256.2019.01.007. |
| [8] |
ZHAO RH, SHI Y, ZHAO H, et al. Acute-on-chronic liver failure in chronic hepatitis B: An update[J]. Expert Rev Gastroenterol Hepatol, 2018, 12( 4): 341- 350. DOI: 10.1080/17474124.2018.1426459. |
| [9] |
KAKISAKA K, KATAOKA K, ONODERA M, et al. Alpha-fetoprotein: A biomarker for the recruitment of progenitor cells in the liver in patients with acute liver injury or failure[J]. Hepatol Res, 2015, 45( 10): E12- E20. DOI: 10.1111/hepr.12448. |
| [10] |
WANG XP, SHEN CF, YANG JJ, et al. Alpha-fetoprotein as a predictive marker for patients with hepatitis B-related acute-on-chronic liver failure[J]. Can J Gastroenterol Hepatol, 2018, 2018: 1232785. DOI: 10.1155/2018/1232785. |
| [11] |
QIN S, TANG SH, WANG XH, et al. Value of serum alpha-fetoprotein for the prognostic evaluation of hepatitis B virus-related acute-on-chronic liver failure treated with artificial liver[J]. Chin J Hepatol, 2020, 28( 1): 69- 72. DOI: 10.3760/cma.j.issn.1007-3418.2020.01.016. |
| [12] |
HOARE M, DAS T, Ageing ALEXANDER G., telomeres, senescence, and injury liver[J]. J Hepatol, 2010, 53( 5): 950- 961. DOI: 10.1016/j.jhep.2010.06.009. |
| [13] |
TANG CL, CHEN H, JIANG L, et al. Liver regeneration: Changes in oxidative stress, immune system, cytokines, and epigenetic modifications associated with aging[J]. Oxid Med Cell Longev, 2022, 2022: 9018811. DOI: 10.1155/2022/9018811. |
| [14] |
ALLAIRE M, GILGENKRANTZ H. The aged liver: Beyond cellular senescence[J]. Clin Res Hepatol Gastroenterol, 2020, 44( 1): 6- 11. DOI: 10.1016/j.clinre.2019.07.011. |
| [15] |
KAMATH PS, WIESNER RH, MALINCHOC M, et al. A model to predict survival in patients with end-stage liver disease[J]. Hepatology, 2001, 33( 2): 464- 470. DOI: 10.1053/jhep.2001.22172. |
| [16] |
LI JQ, LIANG X, YOU SL, et al. Development and validation of a new prognostic score for hepatitis B virus-related acute-on-chronic liver failure[J]. J Hepatol, 2021, 75( 5): 1104- 1115. DOI: 10.1016/j.jhep.2021.05.026. |
| [17] |
MITCHELL O, FELDMAN DM, DIAKOW M, et al. The pathophysiology of thrombocytopenia in chronic liver disease[J]. Hepat Med, 2016, 8: 39- 50. DOI: 10.2147/HMER.S74612. |
| [18] |
XU XW, HOU ZH, XU YY, et al. The dynamic of platelet count as a novel and valuable predictor for 90-day survival of hepatitis B virus-related acute-on-chronic liver failure patients[J]. Clin Res Hepatol Gastroenterol, 2021, 45( 2): 101482. DOI: 10.1016/j.clinre.2020.06.008. |
| [19] |
TU Y, LI X, CHEN MJ, et al. Value of platelet count and related scoring models in predicting the prognosis of hepatitis B virus-related acute-on-chronic liver failure[J]. J Clin Hepatol, 2023, 39( 6): 1308- 1312. DOI: 10.3969/j.issn.1001-5256.2023.06.009. |
| [20] |
ZACCHERINI G, WEISS E, MOREAU R. Acute-on-chronic liver failure: Definitions, pathophysiology and principles of treatment[J]. JHEP Rep, 2021, 3( 1): 100176. DOI: 10.1016/j.jhepr.2020.100176. |
| [21] |
JALAN R, SALIBA F, PAVESI M, et al. Development and validation of a prognostic score to predict mortality in patients with acute-on-chronic liver failure[J]. J Hepatol, 2014, 61( 5): 1038- 1047. DOI: 10.1016/j.jhep.2014.06.012. |
| [22] |
TRIANTAFYLLOU E, WOOLLARD KJ, MCPHAIL MJW, et al. The role of monocytes and macrophages in acute and acute-on-chronic liver failure[J]. Front Immunol, 2018, 9: 2948. DOI: 10.3389/fimmu.2018.02948. |



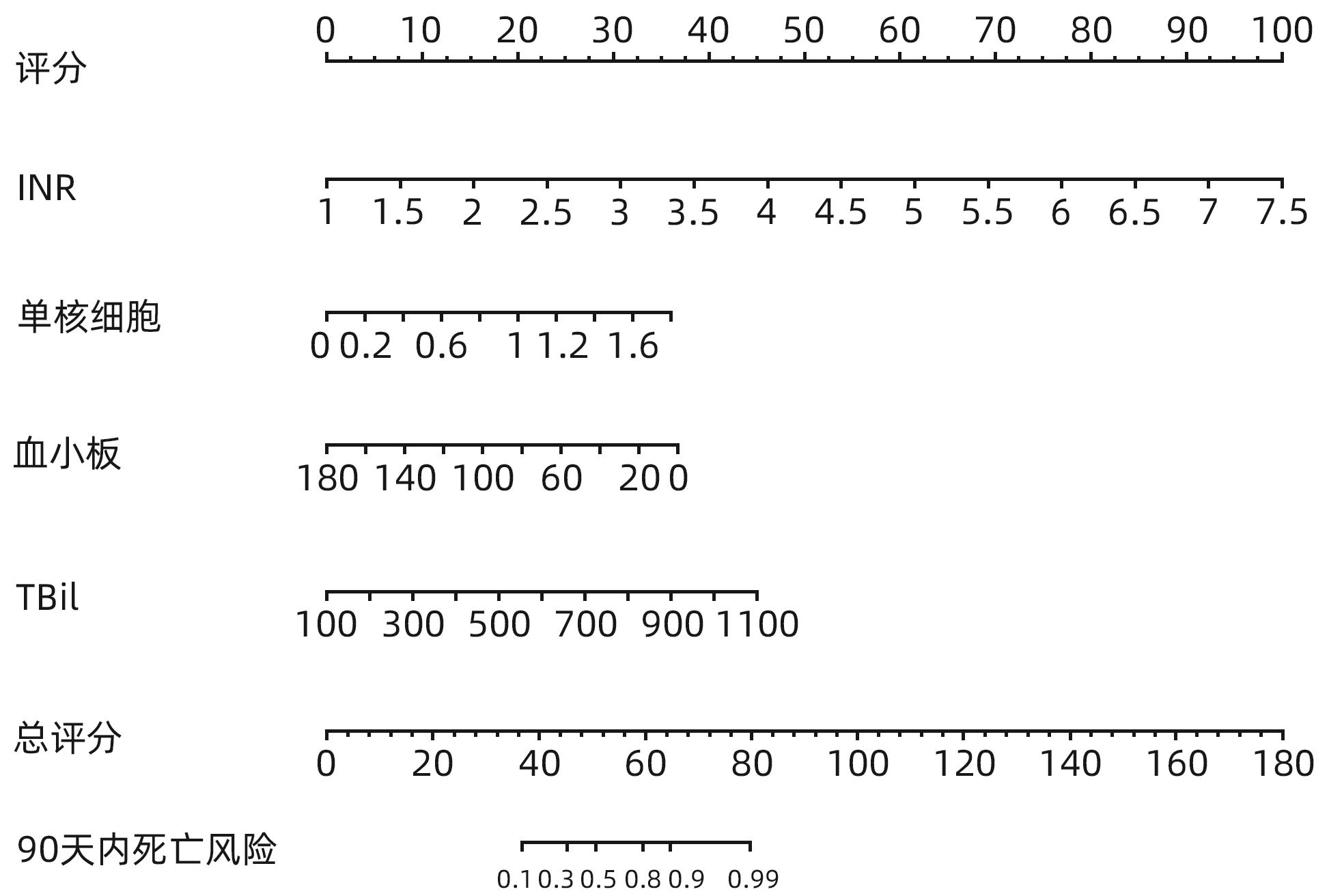




 DownLoad:
DownLoad: