| [1] |
|
| [2] |
SHIFFMAN ML. Autoimmune hepatitis: Epidemiology, subtypes, and presentation[J]. Clin Liver Dis, 2024, 28( 1): 1- 14. DOI: 10.1016/j.cld.2023.06.002. |
| [3] |
MURATORI L, LOHSE AW, LENZI M. Diagnosis and management of autoimmune hepatitis[J]. BMJ, 2023, 380: e070201. DOI: 10.1136/bmj-2022-070201. |
| [4] |
KOMORI A. Recent updates on the management of autoimmune hepatitis[J]. Clin Mol Hepatol, 2021, 27( 1): 58- 69. DOI: 10.3350/cmh.2020.0189. |
| [5] |
PAPE S, SNIJDERS RJALM, GEVERS TJG, et al. Systematic review of response criteria and endpoints in autoimmune hepatitis by the International Autoimmune Hepatitis Group[J]. J Hepatol, 2022, 76( 4): 841- 849. DOI: 10.1016/j.jhep.2021.12.041. |
| [6] |
|
| [7] |
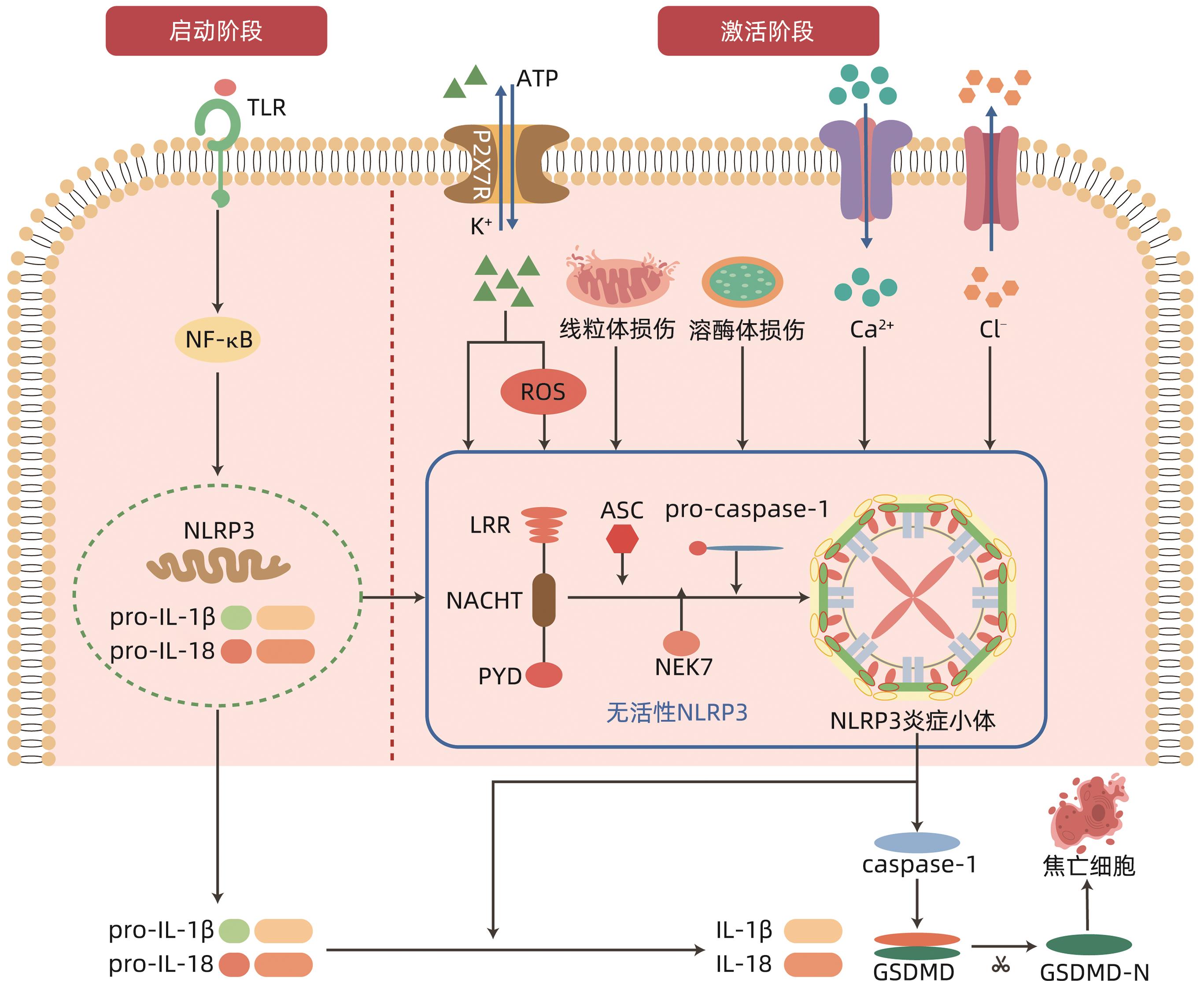
LI Z, GUO JL, BI LQ. Role of the NLRP3 inflammasome in autoimmune diseases[J]. Biomed Pharmacother, 2020, 130: 110542. DOI: 10.1016/j.biopha.2020.110542. |
| [8] |
RUMPRET M, von RICHTHOFEN HJ, PEPERZAK V, et al. Inhibitory pattern recognition receptors[J]. J Exp Med, 2022, 219( 1): e20211463. DOI: 10.1084/jem.20211463. |
| [9] |
LEU SY, TSANG YL, HO LC, et al. NLRP3 inflammasome activation, metabolic danger signals, and protein binding partners[J]. J Endocrinol, 2023, 257( 2): e220184. DOI: 10.1530/JOE-22-0184. |
| [10] |
SCHMIDT FI, LU A, CHEN JW, et al. A single domain antibody fragment that recognizes the adaptor ASC defines the role of ASC domains in inflammasome assembly[J]. J Exp Med, 2016, 213( 5): 771- 790. DOI: 10.1084/jem.20151790. |
| [11] |
NAMBAYAN RJT, SANDIN SI, QUINT DA, et al. The inflammasome adapter ASC assembles into filaments with integral participation of its two Death Domains, PYD and CARD[J]. J Biol Chem, 2019, 294( 2): 439- 452. DOI: 10.1074/jbc.RA118.004407. |
| [12] |
MOLLA MD, AYELIGN B, DESSIE G, et al. Caspase-1 as a regulatory molecule of lipid metabolism[J]. Lipids Health Dis, 2020, 19( 1): 34. DOI: 10.1186/s12944-020-01220-y. |
| [13] |
|
| [14] |
BLEVINS HM, XU YM, BIBY S, et al. The NLRP3 inflammasome pathway: A review of mechanisms and inhibitors for the treatment of inflammatory diseases[J]. Front Aging Neurosci, 2022, 14: 879021. DOI: 10.3389/fnagi.2022.879021. |
| [15] |
BOUCHER D, MONTELEONE M, COLL RC, et al. Caspase-1 self-cleavage is an intrinsic mechanism to terminate inflammasome activity[J]. J Exp Med, 2018, 215( 3): 827- 840. DOI: 10.1084/jem.20172222. |
| [16] |
DUBYAK GR, MILLER BA, PEARLMAN E. Pyroptosis in neutrophils: Multimodal integration of inflammasome and regulated cell death signaling pathways[J]. Immunol Rev, 2023, 314( 1): 229- 249. DOI: 10.1111/imr.13186. |
| [17] |
SWANSON KV, DENG M, TING JPY. The NLRP3 inflammasome: Molecular activation and regulation to therapeutics[J]. Nat Rev Immunol, 2019, 19( 8): 477- 489. DOI: 10.1038/s41577-019-0165-0. |
| [18] |
PELEGRIN P. P2X7 receptor and the NLRP3 inflammasome: Partners in crime[J]. Biochem Pharmacol, 2021, 187: 114385. DOI: 10.1016/j.bcp.2020.114385. |
| [19] |
SHARIF H, WANG L, WANG WL, et al. Structural mechanism for NEK7-licensed activation of NLRP3 inflammasome[J]. Nature, 2019, 570( 7761): 338- 343. DOI: 10.1038/s41586-019-1295-z. |
| [20] |
BERINGER A, MIOSSEC P. IL-17 and IL-17-producing cells and liver diseases, with focus on autoimmune liver diseases[J]. Autoimmun Rev, 2018, 17( 12): 1176- 1185. DOI: 10.1016/j.autrev.2018.06.008. |
| [21] |
BUTCHER MJ, ZHU JF. Recent advances in understanding the Th1/Th2 effector choice[J]. Fac Rev, 2021, 10: 30. DOI: 10.12703/r/10-30. |
| [22] |
WU YN, ZHANG R, SONG XC, et al. C6orf120 gene knockout in rats mitigates concanavalin A-induced autoimmune hepatitis via regulating NKT cells[J]. Cell Immunol, 2022, 371: 104467. DOI: 10.1016/j.cellimm.2021.104467. |
| [23] |
LAN PX, FAN YH, ZHAO Y, et al. TNF superfamily receptor OX40 triggers invariant NKT cell pyroptosis and liver injury[J]. J Clin Invest, 2017, 127( 6): 2222- 2234. DOI: 10.1172/JCI91075. |
| [24] |
SMYK DS, MAVROPOULOS A, MIELI-VERGANI G, et al. The role of invariant NKT in autoimmune liver disease: Can vitamin D act as an immunomodulator?[J]. Can J Gastroenterol Hepatol, 2018, 2018: 8197937. DOI: 10.1155/2018/8197937. |
| [25] |
SIRBE C, SIMU GL, SZABO I, et al. Pathogenesis of autoimmune hepatitis-cellular and molecular mechanisms[J]. Int J Mol Sci, 2021, 22( 24): 13578. DOI: 10.3390/ijms222413578. |
| [26] |
CHRISTEN U, HINTERMANN E. Animal models for autoimmune hepatitis: Are current models good enough?[J]. Front Immunol, 2022, 13: 898615. DOI: 10.3389/fimmu.2022.898615. |
| [27] |
LUAN JY, ZHANG XY, WANG SF, et al. NOD-like receptor protein 3 inflammasome-dependent IL-1β accelerated ConA-induced hepatitis[J]. Front Immunol, 2018, 9: 758. DOI: 10.3389/fimmu.2018.00758. |
| [28] |
LIU ZJ, SUN MY, LIU WH, et al. Deficiency of purinergic P2X4 receptor alleviates experimental autoimmune hepatitis in mice[J]. Biochem Pharmacol, 2024, 221: 116033. DOI: 10.1016/j.bcp.2024.116033. |
| [29] |
WANG H, WANG GD, LIANG YJ, et al. Redox regulation of hepatic NLRP3 inflammasome activation and immune dysregulation in trichloroethene-mediated autoimmunity[J]. Free Radic Biol Med, 2019, 143: 223- 231. DOI: 10.1016/j.freeradbiomed.2019.08.014. |
| [30] |
WANG H, WANG GD, ANSARI GAS, et al. Trichloroethene metabolite dichloroacetyl chloride induces apoptosis and compromises phagocytosis in Kupffer cells: Activation of inflammasome and MAPKs[J]. PLoS One, 2018, 13( 12): e0210200. DOI: 10.1371/journal.pone.0210200. |
| [31] |
WANG KC, WU WR, JIANG XW, et al. Multi-omics analysis reveals the protection of gasdermin D in concanavalin A-induced autoimmune hepatitis[J]. Microbiol Spectr, 2022, 10( 5): e0171722. DOI: 10.1128/spectrum.01717-22. |
| [32] |
GUAN YL, GU YY, LI H, et al. NLRP3 inflammasome activation mechanism and its role in autoimmune liver disease[J]. Acta Biochim Biophys Sin, 2022, 54( 11): 1577- 1586. DOI: 10.3724/abbs.2022137. |
| [33] |
HUANG Y, XU W, ZHOU RB. NLRP3 inflammasome activation and cell death[J]. Cell Mol Immunol, 2021, 18( 9): 2114- 2127. DOI: 10.1038/s41423-021-00740-6. |
| [34] |
XIE HB, PENG JL, ZHANG XS, et al. Effects of mitochondrial reactive oxygen species-induced NLRP3 inflammasome activation on trichloroethylene-mediated kidney immune injury[J]. Ecotoxicol Environ Saf, 2022, 244: 114067. DOI: 10.1016/j.ecoenv.2022.114067. |
| [35] |
LU FB, CHEN DZ, CHEN L, et al. Attenuation of experimental autoimmune hepatitis in mice with bone mesenchymal stem cell-derived exosomes carrying microRNA-223-3p[J]. Mol Cells, 2019, 42( 12): 906- 918. DOI: 10.14348/molcells.2019.2283. |
| [36] |
HUANG C, XING X, XIANG XY, et al. MicroRNAs in autoimmune liver diseases: From diagnosis to potential therapeutic targets[J]. Biomed Pharmacother, 2020, 130: 110558. DOI: 10.1016/j.biopha.2020.110558. |
| [37] |
YU YN, DONG H, ZHANG Y, et al. MicroRNA-223 downregulation promotes HBx-induced podocyte pyroptosis by targeting the NLRP3 inflammasome[J]. Arch Virol, 2022, 167( 9): 1841- 1854. DOI: 10.1007/s00705-022-05499-3. |
| [38] |
LA ROSA F, MANCUSO R, AGOSTINI S, et al. Pharmacological and epigenetic regulators of NLRP3 inflammasome activation in Alzheimer’s disease[J]. Pharmaceuticals, 2021, 14( 11): 1187. DOI: 10.3390/ph14111187. |
| [39] |
LIU D, CHENG HL, LUO JF. Exogenous hydrogen sulfide miR-211-5p targeting TLR4 pathway mitigates liver damage in autoimmune hepatitis mice[J]. Immunol J, 2022, 38( 10): 838- 845. DOI: 10.13431/j.cnki.immunol.j.20220117. |
| [40] |
CHEN L, LU FB, CHEN DZ, et al. BMSCs-derived miR-223-containing exosomes contribute to liver protection in experimental autoimmune hepatitis[J]. Mol Immunol, 2018, 93: 38- 46. DOI: 10.1016/j.molimm.2017.11.008. |
| [41] |
|
| [42] |
COLL RC, SCHRODER K, PELEGRÍN P. NLRP3 and pyroptosis blockers for treating inflammatory diseases[J]. Trends Pharmacol Sci, 2022, 43( 8): 653- 668. DOI: 10.1016/j.tips.2022.04.003. |
| [43] |
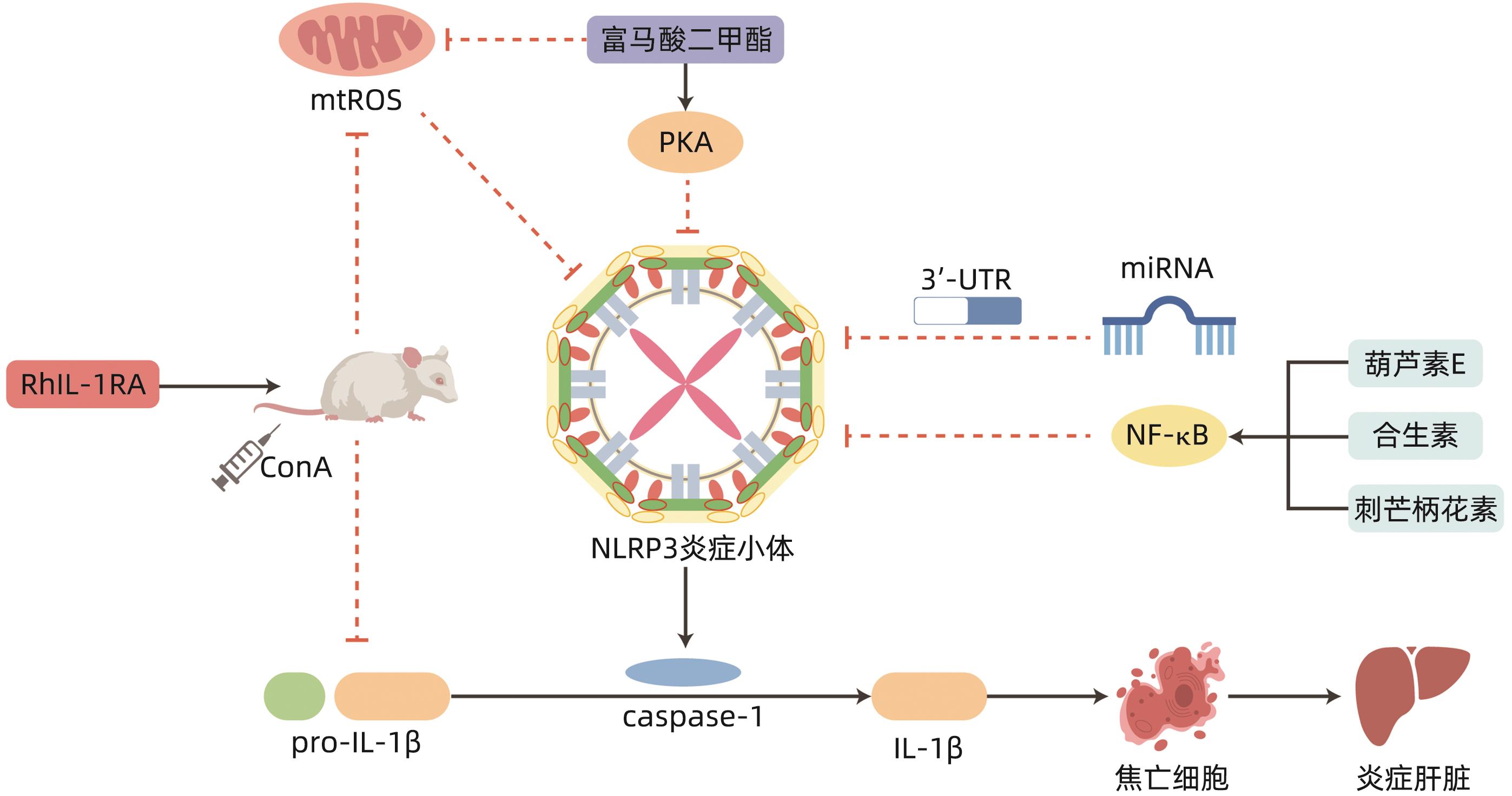
SHI FL, NI ST, LUO SQ, et al. Dimethyl fumarate ameliorates autoimmune hepatitis in mice by blocking NLRP3 inflammasome activation[J]. Int Immunopharmacol, 2022, 108: 108867. DOI: 10.1016/j.intimp.2022.108867. |
| [44] |
SANGINETO M, GRABHERR F, ADOLPH TE, et al. Dimethyl fumarate ameliorates hepatic inflammation in alcohol related liver disease[J]. Liver Int, 2020, 40( 7): 1610- 1619. DOI: 10.1111/liv.14483. |
| [45] |
RAMOS-TOVAR E, MURIEL P. NLRP3 inflammasome in hepatic diseases: A pharmacological target[J]. Biochem Pharmacol, 2023, 217: 115861. DOI: 10.1016/j.bcp.2023.115861. |
| [46] |
LIU GW, ZHAO WX, BAI JM, et al. Formononetin protects against concanavalin-A-induced autoimmune hepatitis in mice through its anti-apoptotic and anti-inflammatory properties[J]. Biochem Cell Biol, 2021, 99( 2): 231- 240. DOI: 10.1139/bcb-2020-0197. |
| [47] |
SILVESTRE GFG, DE LUCENA RP, SILVA ALVES H DA. Cucurbitacins and the immune system: Update in research on anti- inflammatory, antioxidant, and immunomodulatory mechanisms[J]. Curr Med Chem, 2022, 29( 21): 3774- 3789. DOI: 10.2174/0929867329666220107153253. |
| [48] |
MOHAMED GA, IBRAHIM SRM, EL-AGAMY DS, et al. Cucurbitacin E glucoside alleviates concanavalin A-induced hepatitis through enhancing SIRT1/Nrf2/HO-1 and inhibiting NF-κB/NLRP3 signaling pathways[J]. J Ethnopharmacol, 2022, 292: 115223. DOI: 10.1016/j.jep.2022.115223. |
| [49] |
LIU QQ, YANG H, KANG X, et al. A synbiotic ameliorates con A-induced autoimmune hepatitis in mice through modulation of gut microbiota and immune imbalance[J]. Mol Nutr Food Res, 2023, 67( 7): e2200428. DOI: 10.1002/mnfr.202200428. |
| [50] |
KANG YB, KUANG XY, YAN H, et al. A novel synbiotic alleviates autoimmune hepatitis by modulating the gut microbiota-liver axis and inhibiting the hepatic TLR4/NF-κB/NLRP3 signaling pathway[J]. mSystems, 2023, 8( 2): e0112722. DOI: 10.1128/msystems.01127-22. |







 DownLoad:
DownLoad: