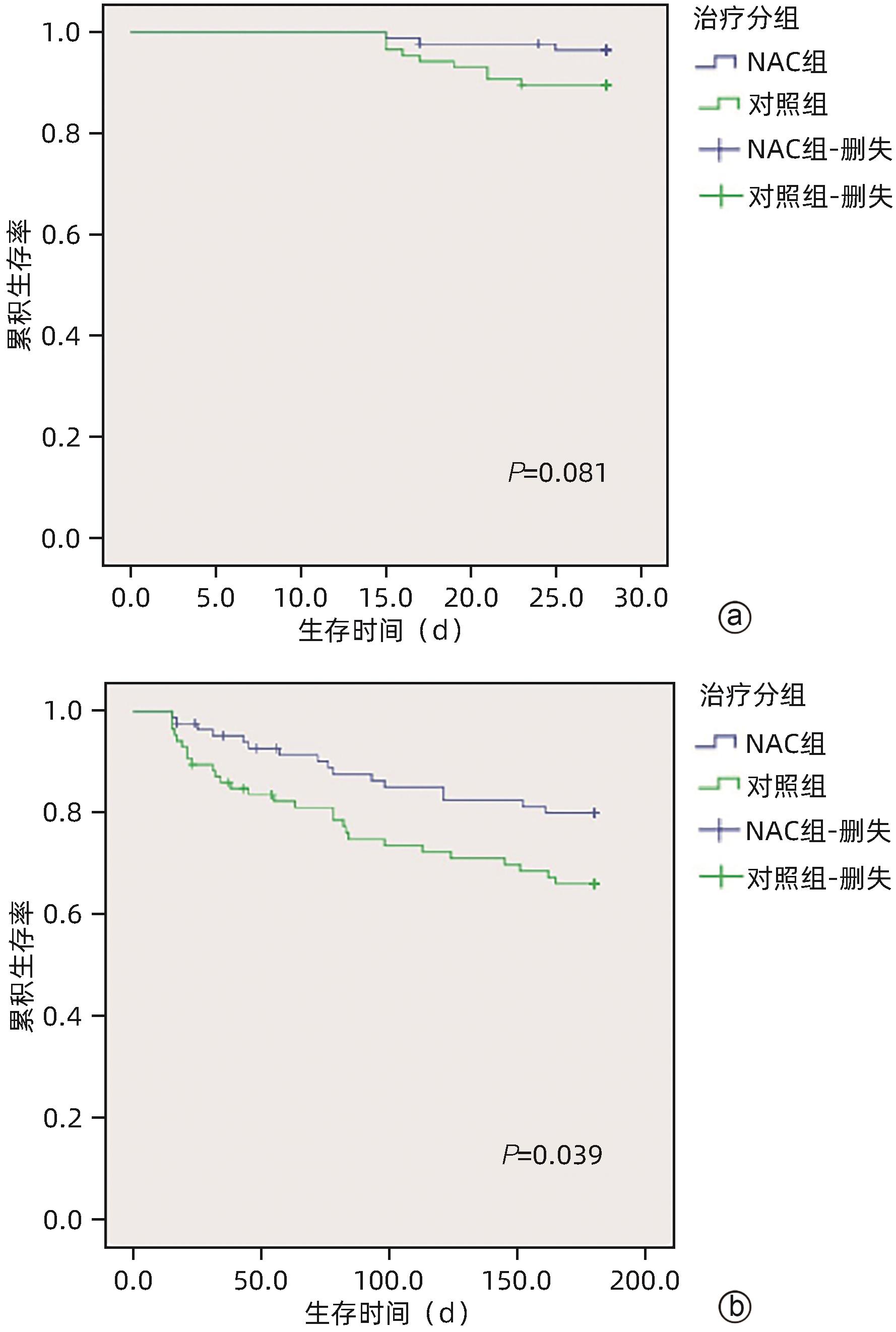
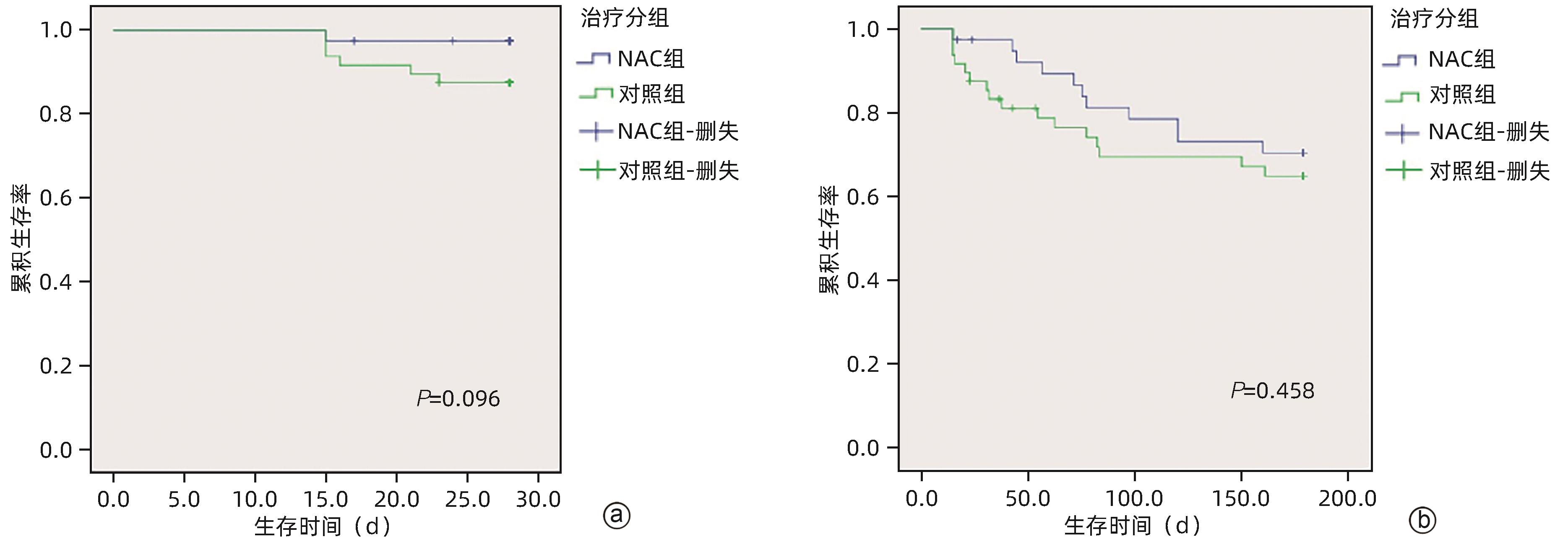
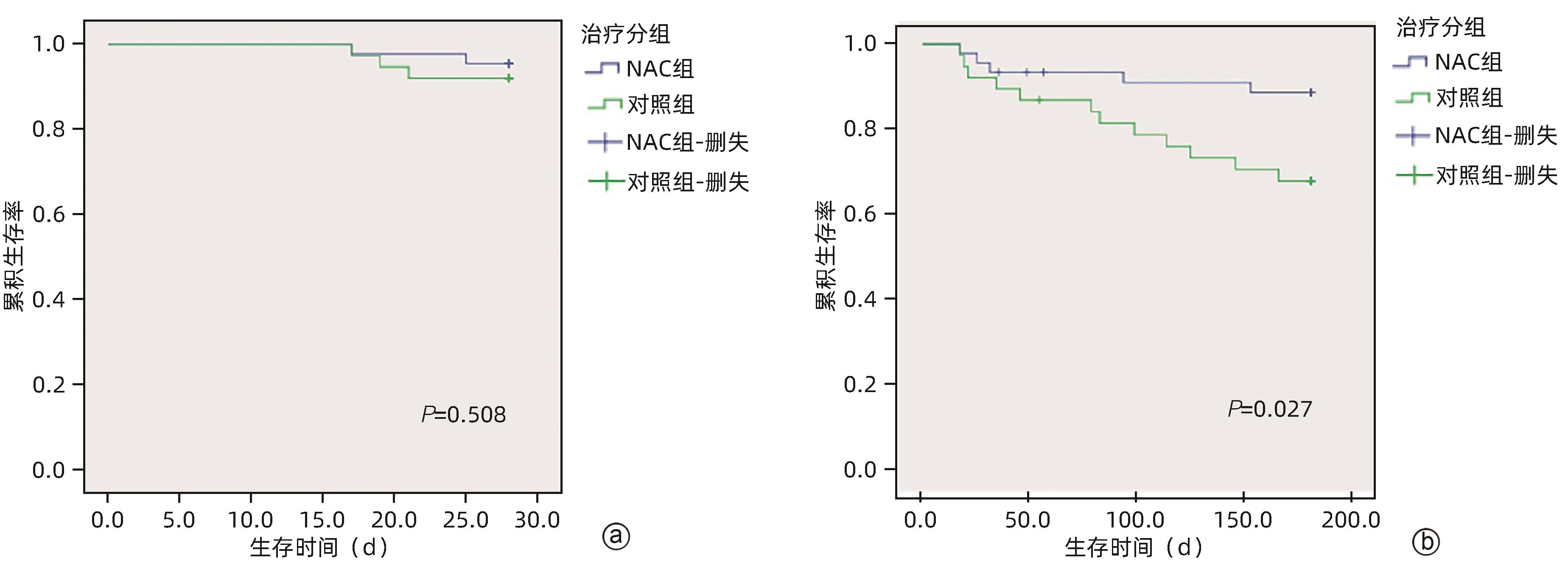
| [1] |
YOU SL, RONG YH, ZHU B, et al. Changing etiology of liver failure in 3, 916 patients from Northern China: A 10-year survey[J]. Hepatol Int, 2013, 7( 2): 714- 720. DOI: 10.1007/s12072-013-9424-5. |
| [2] |
ZHU B, YOU SL, LIU HL, et al. Analysis of etiology and prognosis of liver failure in 623 elderly patients[J]. Med J Chin People’s Liberation Army, 2014, 39( 8): 624- 627. DOI: 10.11855/j.issn.0577-7402.2014.08.06. |
| [3] |
LIU JJ, ZHANG MY, SUN YM, et al. Evolution in the indications for anti-viral therapy in chronic hepatitis B[J]. J Clin Hepatol, 2023, 39( 6): 1299- 1303. DOI: 10.3969/j.issn.1001-5256.2023.06.007. |
| [4] |
CAO XZ, YI W, WANG FC, et al. Mother-to-child blockade with telbivudine of pregnant women infected with hepatitis B virus and the influence on the immune response to hepatitis B vaccine of infants[J/CD]. Chin J Exp Clin Infect Dis(Electronic Edition), 2022, 16( 3): 158- 164. DOI: 10.3877/cma.j.issn.1674-1358.2022.03.003. |
| [5] |
|
| [6] |
European Association for the Study of the Liver. EASL clinical practice guidelines: Management of alcohol-related liver disease[J]. J Hepatol, 2018, 69( 1): 154- 181. DOI: 10.1016/j.jhep.2018.03.018. |
| [7] |
RAMOND MJ, POYNARD T, RUEFF B, et al. A randomized trial of prednisolone in patients with severe alcoholic hepatitis[J]. N Engl J Med, 1992, 326( 8): 507- 512. DOI: 10.1056/NEJM199202203260802. |
| [8] |
SINGAL AK, BATALLER R, AHN J, et al. ACG clinical guideline: Alcoholic liver disease[J]. Am J Gastroenterol, 2018, 113( 2): 175- 194. DOI: 10.1038/ajg.2017.469. |
| [9] |
Liver Failure and Artificial Liver Group, Chinese Society of Infectious Diseases, Chinese Medical Association; Severe Liver Disease and Artificial Liver Group, Chinese Society of Hepatology, Chinese Medical Association. Guideline for diagnosis and treatment of liver failure(2018)[J]. J Clin Hepatol, 2019, 35( 1): 38- 44. DOI: 10.3969/j.issn.1001-5256.2019.01.007. |
| [10] |
MATHURIN P, O’GRADY J, CARITHERS RL, et al. Corticosteroids improve short-term survival in patients with severe alcoholic hepatitis: Meta-analysis of individual patient data[J]. Gut, 2011, 60( 2): 255- 260. DOI: 10.1136/gut.2010.224097. |
| [11] |
THURSZ MR, RICHARDSON P, ALLISON M, et al. Prednisolone or pentoxifylline for alcoholic hepatitis[J]. N Engl J Med, 2015, 372( 17): 1619- 1628. DOI: 10.1056/NEJMoa1412278. |
| [12] |
LOUVET A, WARTEL F, CASTEL H, et al. Infection in patients with severe alcoholic hepatitis treated with steroids: Early response to therapy is the key factor[J]. Gastroenterology, 2009, 137( 2): 541- 548. DOI: 10.1053/j.gastro.2009.04.062. |
| [13] |
MATHURIN P, LOUVET A, DUHAMEL A, et al. Prednisolone with vs without pentoxifylline and survival of patients with severe alcoholic hepatitis: A randomized clinical trial[J]. JAMA, 2013, 310( 10): 1033- 1041. DOI: 10.1001/jama.2013.276300. |
| [14] |
YANG YM, CHO YE, HWANG S. Crosstalk between oxidative stress and inflammatory liver injury in the pathogenesis of alcoholic liver disease[J]. Int J Mol Sci, 2022, 23( 2): 774. DOI: 10.3390/ijms23020774. |
| [15] |
DEY A, CEDERBAUM AI. Alcohol and oxidative liver injury[J]. Hepatology, 2006, 43(2 Suppl 1): S63- S74. DOI: 10.1002/hep.20957. |
| [16] |
NGUYEN-KHAC E, THEVENOT T, PIQUET MA, et al. Glucocorticoids plus N-acetylcysteine in severe alcoholic hepatitis[J]. N Engl J Med, 2011, 365( 19): 1781- 1789. DOI: 10.1056/NEJMoa1101214. |
| [17] |
MORENO C, LANGLET P, HITTELET A, et al. Enteral nutrition with or without N-acetylcysteine in the treatment of severe acute alcoholic hepatitis: A randomized multicenter controlled trial[J]. J Hepatol, 2010, 53( 6): 1117- 1122. DOI: 10.1016/j.jhep.2010.05.030. |
| [18] |
STEWART S, PRINCE M, BASSENDINE M, et al. A randomized trial of antioxidant therapy alone or with corticosteroids in acute alcoholic hepatitis[J]. J Hepatol, 2007, 47( 2): 277- 283. DOI: 10.1016/j.jhep.2007.03.027. |
| [19] |
PHILLIPS M, CURTIS H, PORTMANN B, et al. Antioxidants versus corticosteroids in the treatment of severe alcoholic hepatitis: A randomised clinical trial[J]. J Hepatol, 2006, 44( 4): 784- 790. DOI: 10.1016/j.jhep.2005.11.039. |
| [20] |
MUMTAZ K, AZAM Z, HAMID S, et al. Role of N-acetylcysteine in adults with non-acetaminophen-induced acute liver failure in a center without the facility of liver transplantation[J]. Hepatol Int, 2009, 3( 4): 563- 570. DOI: 10.1007/s12072-009-9151-0. |
| [21] |
NABI T, NABI S, RAFIQ N, et al. Role of N-acetylcysteine treatment in non-acetaminophen-induced acute liver failure: A prospective study[J]. Saudi J Gastroenterol, 2017, 23( 3): 169- 175. DOI: 10.4103/1319-3767.207711. |



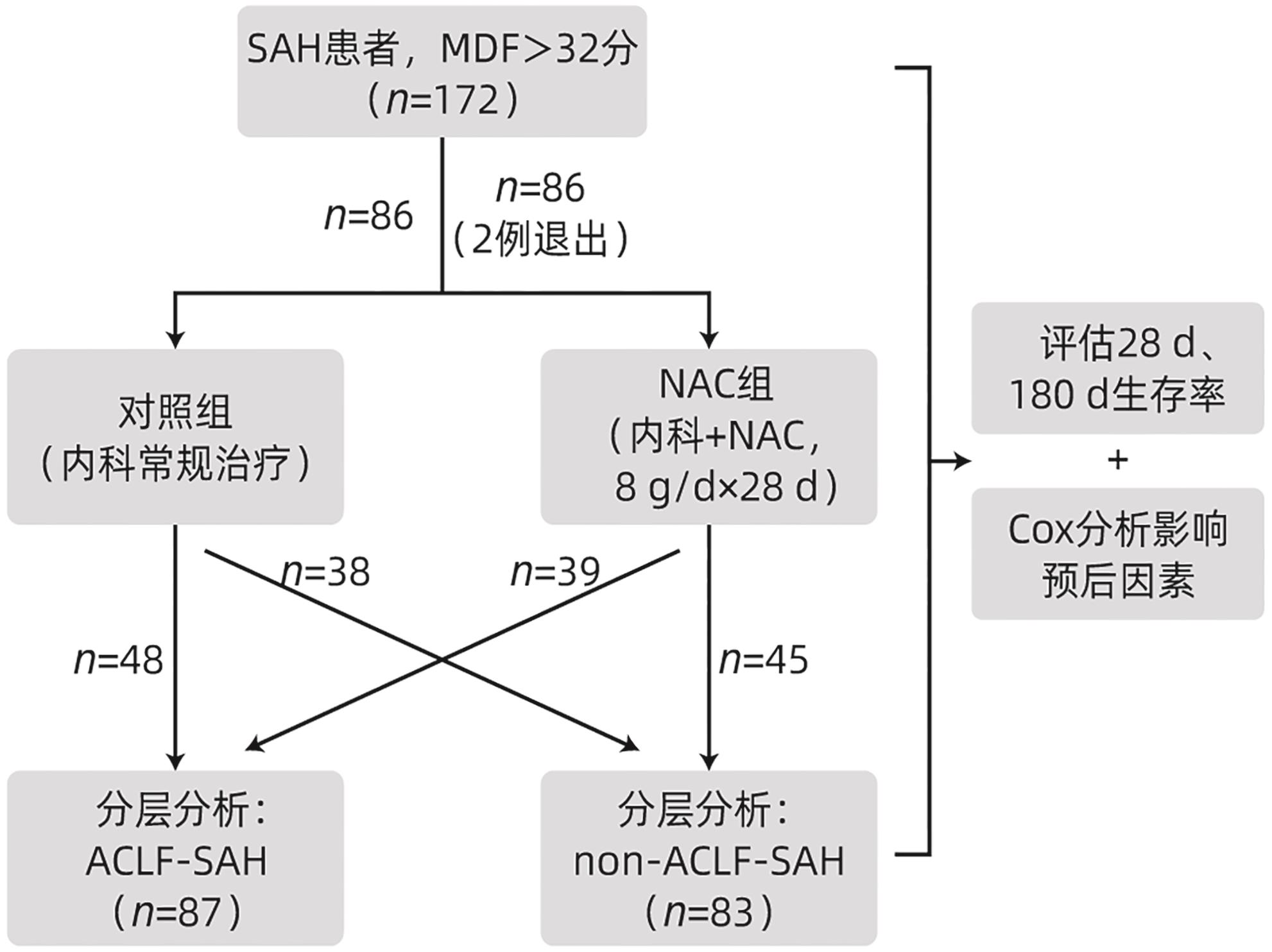




 DownLoad:
DownLoad: