| [1] |
Technology Committee on DILI Prevention and Management, Chinese Medical Biotechnology Association; Study Group of Drug-Induced Liver Disease, Chinese Medical Association for the Study of Liver Diseases. Chinese guideline for diagnosis and management of drug-induced liver injury(2023 version)[J]. Chin J Gastroenterol, 2023, 28( 7): 397- 431. DOI: 10.3760/cma.j.cn501113-20230419-00176. |
| [2] |
RANI J, DHULL SB, ROSE PK, et al. Drug-induced liver injury and anti-hepatotoxic effect of herbal compounds: A metabolic mechanism perspective[J]. Phytomedicine, 2024, 122: 155142. DOI: 10.1016/j.phymed.2023.155142. |
| [3] |
SINGH S, KUMAR PVSNK, KUMAR JP, et al. Genetic and epigenetic basis of drug-induced liver injury[J]. Semin Liver Dis, 2023, 43( 2): 163- 175. DOI: 10.1055/a-2097-0531. |
| [4] |
ZHANG D, HAO JQ, HOU RL, et al. The role of NAT2 polymorphism and methylation in anti-tuberculosis drug-induced liver injury in Mongolian tuberculosis patients[J]. J Clin Pharm Ther, 2020, 45( 3): 561- 569. DOI: 10.1111/jcpt.13097. |
| [5] |
DEVARBHAVI H, PATIL M, MENON M. Association of human leukocyte antigen-B*13:01 with dapsone-induced liver injury[J]. Br J Clin Pharmacol, 2022, 88( 3): 1369- 1372. DOI: 10.1111/bcp.15054. |
| [6] |
ASIF BA, KOH C, PHILLIPS EJ, et al. Vancomycin-induced liver injury, DRESS, and HLA-a 32:01[J]. J Allergy Clin Immunol Pract, 2024, 12( 1): 168- 174. DOI: 10.1016/j.jaip.2023.09.011. |
| [7] |
NICOLETTI P, DELLINGER A, LI YJ, et al. Identification of reduced ERAP2 expression and a novel HLA allele as components of a risk score for susceptibility to liver injury due to amoxicillin-clavulanate[J]. Gastroenterology, 2023, 164( 3): 454- 466. DOI: 10.1053/j.gastro.2022.11.036. |
| [8] |
JING J, HE TT, BAI ZF, et al. An excerpt from AASLD Practice Guidance on drug, herbal and dietary supplement-induced liver injury[J]. J Clin Hepatol, 2022, 38( 10): 2219- 2223 DOI: 10.3969/j.issn.1001-5256.2022.10.005. |
| [9] |
HOOFNAGLE JH, BONKOVSKY HL, PHILLIPS EJ, et al. HLA-B*35:01 and green tea-induced liver injury[J]. Hepatology, 2021, 73( 6): 2484- 2493. DOI: 10.1002/hep.31538. |
| [10] |
SANTOS EA, GONÇALVES JCS, FLEURY MK, et al. Relationship of anti-tuberculosis drug-induced liver injury and genetic polymorphisms in CYP2E1 and GST[J]. Braz J Infect Dis, 2019, 23( 6): 381- 387. DOI: 10.1016/j.bjid.2019.09.003. |
| [11] |
CHEN SX, PAN HQ, CHEN YZ, et al. Association between genetic polymorphisms of NRF2, KEAP1, MAFF, MAFK and anti-tuberculosis drug-induced liver injury: A nested case-control study[J]. Sci Rep, 2019, 9( 1): 14311. DOI: 10.1038/s41598-019-50706-y. |
| [12] |
KOIDO M, KAWAKAMI E, FUKUMURA J, et al. Polygenic architecture informs potential vulnerability to drug-induced liver injury[J]. Nat Med, 2020, 26( 10): 1541- 1548. DOI: 10.1038/s41591-020-1023-0. |
| [13] |
ANTOINE DJ, WILLIAMS DP, KIPAR A, et al. High-mobility group box-1 protein and keratin-18, circulating serum proteins informative of acetaminophen-induced necrosis and apoptosis in vivo[J]. Toxicol Sci, 2009, 112( 2): 521- 531. DOI: 10.1093/toxsci/kfp235. |
| [14] |
LIU ZC, WANG YP, BORLAK J, et al. Mechanistically linked serum miRNAs distinguish between drug induced and fatty liver disease of different grades[J]. Sci Rep, 2016, 6: 23709. DOI: 10.1038/srep23709. |
| [15] |
FROMENTY B. Alteration of mitochondrial DNA homeostasis in drug-induced liver injury[J]. Food Chem Toxicol, 2020, 135: 110916. DOI: 10.1016/j.fct.2019.110916. |
| [16] |
CHONG YZ, ZHU HY, REN Q, et al. Interaction between the HIF-1α gene rs1957757 polymorphism and CpG island methylation in the promoter region is associated with the risk of anti-tuberculosis drug-induced liver injury in humans: A case-control study[J]. J Clin Pharm Ther, 2022, 47( 7): 948- 955. DOI: 10.1111/jcpt.13625. |
| [17] |
WEI YQ, HUAI C, ZHOU CX, et al. A methylation functional detection hepatic cell system validates correlation between DNA methylation and drug-induced liver injury[J]. Pharmacogenomics J, 2020, 20( 5): 717- 723. DOI: 10.1038/s41397-020-0160-7. |
| [18] |
LAMMERT C, ZHU CS, LIAN Y, et al. Exploratory study of autoantibody profiling in drug-induced liver injury with an autoimmune phenotype[J]. Hepatol Commun, 2020, 4( 11): 1651- 1663. DOI: 10.1002/hep4.1582. |
| [19] |
LEE SK, CHOI JY, JUNG ES, et al. An immunological perspective on the mechanism of drug induced liver injury: Focused on drugs for treatment of hepatocellular carcinoma and liver transplantation[J]. Int J Mol Sci, 2023, 24( 5): 5002. DOI: 10.3390/ijms24055002. |
| [20] |
Food and Drug Allergy Prevention Subgroup, Allergy Prevention and Control Committee of Chinese Preventive Medicine Association. Chinese expert consensus on approach to the diagnosis and prevention of drug allergy[J]. Chin J Prev Med, 2022, 56( 6): 682- 706. DOI: 10.3760/cma.j.cn112150-20220129-00100. |
| [21] |
TAN CK, HO D, WANG LM, et al. Drug-induced autoimmune hepatitis: A minireview[J]. World J Gastroenterol, 2022, 28( 24): 2654- 2666. DOI: 10.3748/wjg.v28.i24.2654. |
| [22] |
|
| [23] |
BJÖRNSSON HK, GUDBJORNSSON B, BJÖRNSSON ES. Infliximab-induced liver injury: Clinical phenotypes, autoimmunity and the role of corticosteroid treatment[J]. J Hepatol, 2022, 76( 1): 86- 92. DOI: 10.1016/j.jhep.2021.08.024. |
| [24] |
WEBER S, BENESIC A, BUCHHOLTZ ML, et al. Antimitochondrial rather than antinuclear antibodies correlate with severe drug-induced liver injury[J]. Dig Dis, 2021, 39( 3): 275- 282. DOI: 10.1159/000511635. |
| [25] |
YAN MZ, ZHAO C, LU SY, et al. Trimethylamine N-oxide exacerbates acetaminophen-induced liver injury by interfering with macrophage-mediated liver regeneration[J]. J Cell Physiol, 2022, 237( 1): 897- 910. DOI: 10.1002/jcp.30568. |
| [26] |
BOETTLER T, CSERNALABICS B, SALIÉ H, et al. SARS-CoV-2 vaccination can elicit a CD8 T-cell dominant hepatitis[J]. J Hepatol, 2022, 77( 3): 653- 659. DOI: 10.1016/j.jhep.2022.03.040. |
| [27] |
MONSHI MM, FAULKNER L, GIBSON A, et al. Human leukocyte antigen(HLA)-B*57: 01-restricted activation of drug-specific T cells provides the immunological basis for flucloxacillin-induced liver injury[J]. Hepatology, 2013, 57( 2): 727- 739. DOI: 10.1002/hep.26077. |
| [28] |
PUIG M, ANANTHULA S, VENNA R, et al. Alterations in the HLA-B*57:01 immunopeptidome by flucloxacillin and immunogenicity of drug-haptenated peptides[J]. Front Immunol, 2021, 11: 629399. DOI: 10.3389/fimmu.2020.629399. |
| [29] |
ANANTHULA S, KRISHNAVENI SIVAKUMAR K, CARDONE M, et al. Development of mouse models with restricted HLA-B 57: 01 presentation for the study of flucloxacillin-driven T-cell activation and tolerance in liver injury[J]. J Allergy Clin Immunol, 2023, 152( 2): 486- 499. e 7. DOI: 10.1016/j.jaci.2023.03.029. |
| [30] |
HERNANDEZ N, BESSONE F. Hepatotoxicity induced by biological agents: Clinical features and current controversies[J]. J Clin Transl Hepatol, 2022, 10( 3): 486- 495. DOI: 10.14218/JCTH.2021.00243. |
| [31] |
FERNANDEZ-SANTAMARIA R, ARIZA A, FERNANDEZ TD, et al. Advances and highlights in T and B cell responses to drug antigens[J]. Allergy, 2022, 77( 4): 1129- 1138. DOI: 10.1111/all.15126. |
| [32] |
LI DP, CHEN Y, WAN MJ, et al. Oral magnesium prevents acetaminophen-induced acute liver injury by modulating microbial metabolism[J]. Cell Host Microbe, 2024, 32( 1): 48- 62. DOI: 10.1016/j.chom.2023.11.006. |
| [33] |
ZENG YN, WU R, WANG FZ, et al. Liberation of daidzein by gut microbial β-galactosidase suppresses acetaminophen-induced hepatotoxicity in mice[J]. Cell Host Microbe, 2023, 31( 5): 766- 780. DOI: 10.1016/j.chom.2023.04.002. |
| [34] |
SUN MD, CHEN PY, XIAO K, et al. Circulating cell-free DNAs as a biomarker and therapeutic target for acetaminophen-induced liver injury[J]. Adv Sci, 2023, 10( 16): e2206789. DOI: 10.1002/advs.202206789. |
| [35] |
ROTH RA, MAIURI AR, GANEY PE. Idiosyncratic drug-induced liver injury: Is drug-cytokine interaction the linchpin?[J]. J Pharmacol Exp Ther, 2017, 360( 2): 461- 470. DOI: 10.1124/jpet.116.237578. |
| [36] |
LAI RT, XIANG XG, MO RD, et al. Protective effect of Th22 cells and intrahepatic IL-22 in drug induced hepatocellular injury[J]. J Hepatol, 2015, 63( 1): 148- 155. DOI: 10.1016/j.jhep.2015.02.004. |
| [37] |
WANG XF, SUN R, CHEN YY, et al. Regulatory T cells ameliorate acetaminophen-induced immune-mediated liver injury[J]. Int Immunopharmacol, 2015, 25( 2): 293- 301. DOI: 10.1016/j.intimp.2015.02.008. |
| [38] |
WANG Y, LI ZY, LI S, et al. Research advances in adverse liver reactions caused by immune checkpoint inhibitors[J]. J Clin Hepatol, 2022, 38( 1): 220- 223. DOI: 10.3969/j.issn.1001-5256.2022.01.039. |
| [39] |
CUNNINGHAM M, GUPTA R, BUTLER M. Checkpoint inhibitor hepatotoxicity: Pathogenesis and management[J]. Hepatology, 2024, 79( 1): 198- 212. DOI: 10.1097/HEP.0000000000000045. |



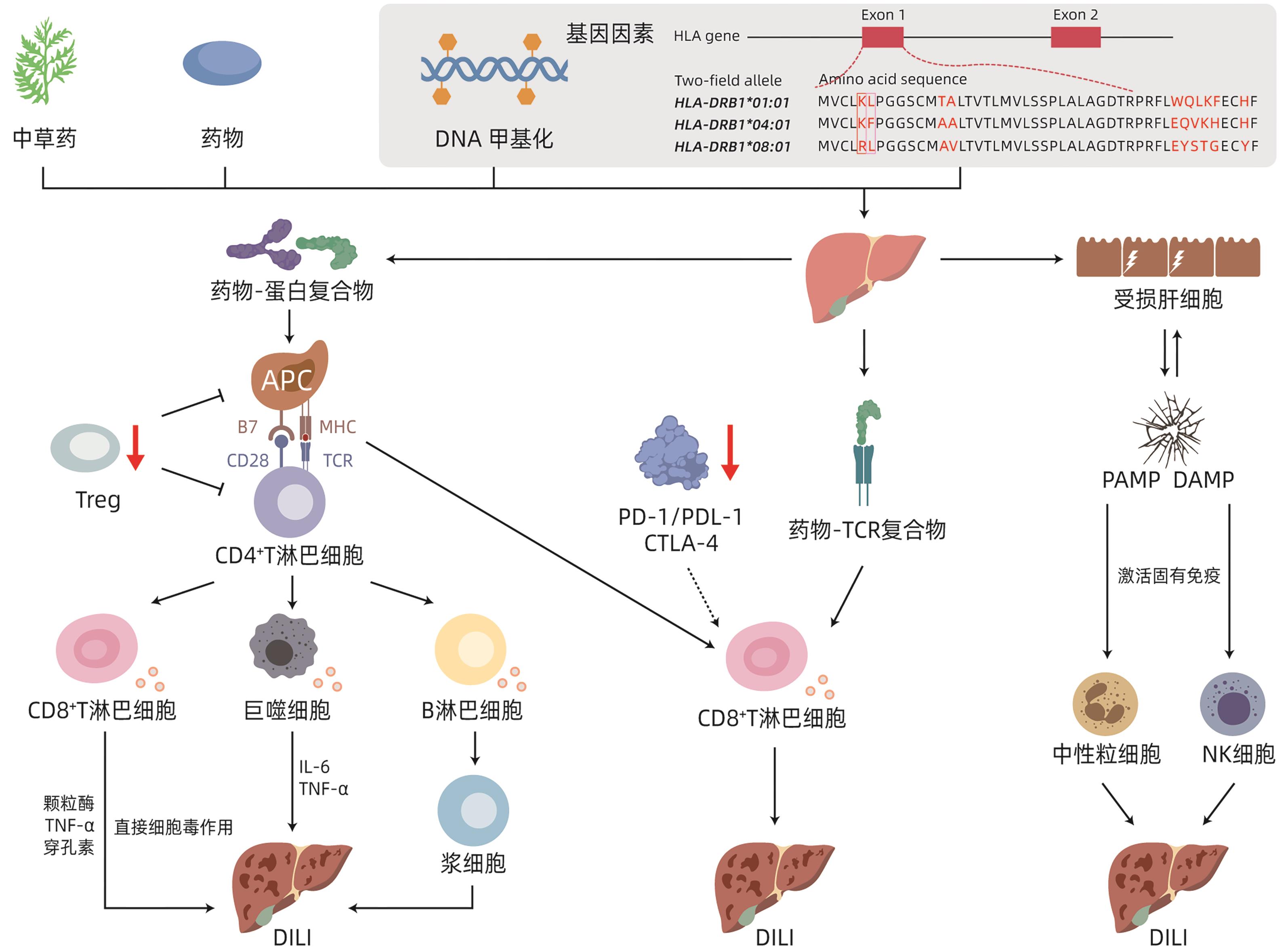




 DownLoad:
DownLoad: