| [1] |
LIU MH, LIU ST, ZHANG LH, et al. Mechanism of ferroptosis in the formation of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis[J]. J Clin Hepatol, 2022, 38( 5): 1152- 1155. DOI: 10.3969/j.issn.1001-5256.2022.05.037. |
| [2] |
RONG L, ZOU JY, RAN W, et al. Advancements in the treatment of non-alcoholic fatty liver disease(NAFLD)[J]. Front Endocrinol(Lausanne), 2023, 13: 1087260. DOI: 10.3389/fendo.2022.1087260. |
| [3] |
RIAZI K, AZHARI H, CHARETTE JH, et al. The prevalence and incidence of NAFLD worldwide: A systematic review and meta-analysis[J]. Lancet Gastroenterol Hepatol, 2022, 7( 9): 851- 861. DOI: 10.1016/S2468-1253(22)00165-0. |
| [4] |
YOUNOSSI ZM, STEPANOVA M, ONG J, et al. Nonalcoholic steatohepatitis is the most rapidly increasing indication for liver transplantation in the United States[J]. Clin Gastroenterol Hepatol, 2021, 19( 3): 580- 589. DOI: 10.1016/j.cgh.2020.05.064. |
| [5] |
HE SH, DAI L, ZHENG J, et al. Therapeutic effect of low-carbohydrate diet and lifestyle intervention on patients with lean nonalcoholic fatty liver disease[J]. J Clin Hepatol, 2024, 40( 5): 946- 951. DOI: 10.12449/JCH240513. 何诗华, 戴璐, 郑洁, 等. 低碳水化合物饮食和生活方式干预对瘦型非酒精性脂肪性肝病患者的疗效观察[J]. 临床肝胆病杂志, 2024, 40( 5): 946- 951. DOI: 10.12449/JCH240513. |
| [6] |
GOLABI P, PAIK J, FUKUI N, et al. Patients with lean nonalcoholic fatty liver disease are metabolically abnormal and have a higher risk for mortality[J]. Clin Diabetes, 2019, 37( 1): 65- 72. DOI: 10.2337/cd18-0026. |
| [7] |
NABI O, LAPIDUS N, BOURSIER J, et al. Lean individuals with NAFLD have more severe liver disease and poorer clinical outcomes(NASH-CO Study)[J]. Hepatology, 2023, 78( 1): 272- 283. DOI: 10.1097/HEP.0000000000000329. |
| [8] |
SU YS, CHEN YW. AGA clinical practice update: diagnosis and management of nonalcoholic fatty liver disease in lean individuals: expert review[J]. Chin J Gastroenterol Hepatol, 2024, 33( 3): 324- 331. DOI: 10.3969/j.issn.1006-5709.2024.03.018. |
| [9] |
FAHIM SM, CHOWDHURY MAB, ALAM S. Non-alcoholic fatty liver disease(NAFLD) among underweight adults[J]. Clin Nutr ESPEN, 2020, 38: 80- 85. DOI: 10.1016/j.clnesp.2020.06.002. |
| [10] |
VILARINHO S, AJMERA V, ZHENG M, et al. Emerging role of genomic analysis in clinical evaluation of lean individuals with NAFLD[J]. Hepatology, 2021, 74( 4): 2241- 2250. DOI: 10.1002/hep.32047. |
| [11] |
|
| [12] |
CHAHAL D, SHARMA D, KESHAVARZI S, et al. Distinctive clinical and genetic features of lean vs overweight fatty liver disease using the UK Biobank[J]. Hepatol Int, 2022, 16( 2): 325- 336. DOI: 10.1007/s12072-022-10304-z. |
| [13] |
ISAC T, ISAC S, RABABOC R, et al. Epigenetics in inflammatory liver diseases: A clinical perspective(Review)[J]. Exp Ther Med, 2022, 23( 5): 366. DOI: 10.3892/etm.2022.11293. |
| [14] |
SHI YC, ZHANG HJ, HUANG SL, et al. Epigenetic regulation in cardiovascular disease: Mechanisms and advances in clinical trials[J]. Signal Transduct Target Ther, 2022, 7( 1): 200. DOI: 10.1038/s41392-022-01055-2. |
| [15] |
MOORE LD, LE T, FAN GP. DNA methylation and its basic function[J]. Neuropsychopharmacology, 2013, 38( 1): 23- 38. DOI: 10.1038/npp.2012.112. |
| [16] |
YANG ZH, DANG YQ, JI G. Role of epigenetics in transformation of inflammation into colorectal cancer[J]. World J Gastroenterol, 2019, 25( 23): 2863- 2877. DOI: 10.3748/wjg.v25.i23.2863. |
| [17] |
GONZÁLEZ-BENGTSSON A, ASADI A, GAO H, et al. Estrogen enhances the expression of the polyunsaturated fatty acid elongase Elovl2 via ERα in breast cancer cells[J]. PLoS One, 2016, 11( 10): e0164241. DOI: 10.1371/journal.pone.0164241. |
| [18] |
LI X, WANG JQ, WANG LY, et al. Lipid metabolism dysfunction induced by age-dependent DNA methylation accelerates aging[J]. Signal Transduct Target Ther, 2022, 7( 1): 162. DOI: 10.1038/s41392-022-00964-6. |
| [19] |
PAUTER AM, OLSSON P, ASADI A, et al. Elovl2 ablation demonstrates that systemic DHA is endogenously produced and is essential for lipid homeostasis in mice[J]. J Lipid Res, 2014, 55( 4): 718- 728. DOI: 10.1194/jlr.M046151. |
| [20] |
LI X, LI XD. Integrative chemical biology approaches to deciphering the histone code: A problem-driven journey[J]. Acc Chem Res, 2021, 54( 19): 3734- 3747. DOI: 10.1021/acs.accounts.1c00463. |
| [21] |
GIALLONGO S, LO RE O, LOCHMANOVÁ G, et al. Phosphorylation within intrinsic disordered region discriminates histone variant macroH2A1 splicing isoforms-macroH2A1.1 and macroH2A1.2[J]. Biology(Basel), 2021, 10( 7): 659. DOI: 10.3390/biology10070659. |
| [22] |
BUZOVA D, MAUGERI A, LIGUORI A, et al. Circulating histone signature of human lean metabolic-associated fatty liver disease(MAFLD)[J]. Clin Epigenetics, 2020, 12( 1): 126. DOI: 10.1186/s13148-020-00917-2. |
| [23] |
HOLOCH D, MOAZED D. RNA-mediated epigenetic regulation of gene expression[J]. Nat Rev Genet, 2015, 16( 2): 71- 84. DOI: 10.1038/nrg3863. |
| [24] |
AMERIKANOU C, PAPADA E, GIOXARI A, et al. Mastiha has efficacy in immune-mediated inflammatory diseases through a microRNA-155 Th17 dependent action[J]. Pharmacol Res, 2021, 171: 105753. DOI: 10.1016/j.phrs.2021.105753. |
| [25] |
SHEN N, TANG L, QIAN YF, et al. Serum miR-4488 as a potential biomarker of lean nonalcoholic fatty liver disease[J]. Ann Transl Med, 2023, 11( 4): 173. DOI: 10.21037/atm-22-6620. |
| [26] |
DAI L, XU JJ, LIU BC, et al. Lingguizhugan Decoction, a Chinese herbal formula, improves insulin resistance in overweight/obese subjects with non-alcoholic fatty liver disease: A translational approach[J]. Front Med, 2022, 16( 5): 745- 759. DOI: 10.1007/s11684-021-0880-3. |
| [27] |
HYMEL E, FISHER KW, FARAZI PA. Differential methylation patterns in lean and obese non-alcoholic steatohepatitis-associated hepatocellular carcinoma[J]. BMC Cancer, 2022, 22( 1): 1276. DOI: 10.1186/s12885-022-10389-7. |
| [28] |
LI DD, LIU Y, XUE L, et al. Up-regulation of microRNA-367 promotes liver steatosis through repressing TBL1 in obese mice[J]. Eur Rev Med Pharmacol Sci, 2017, 21( 7): 1598- 1603.
|
| [29] |
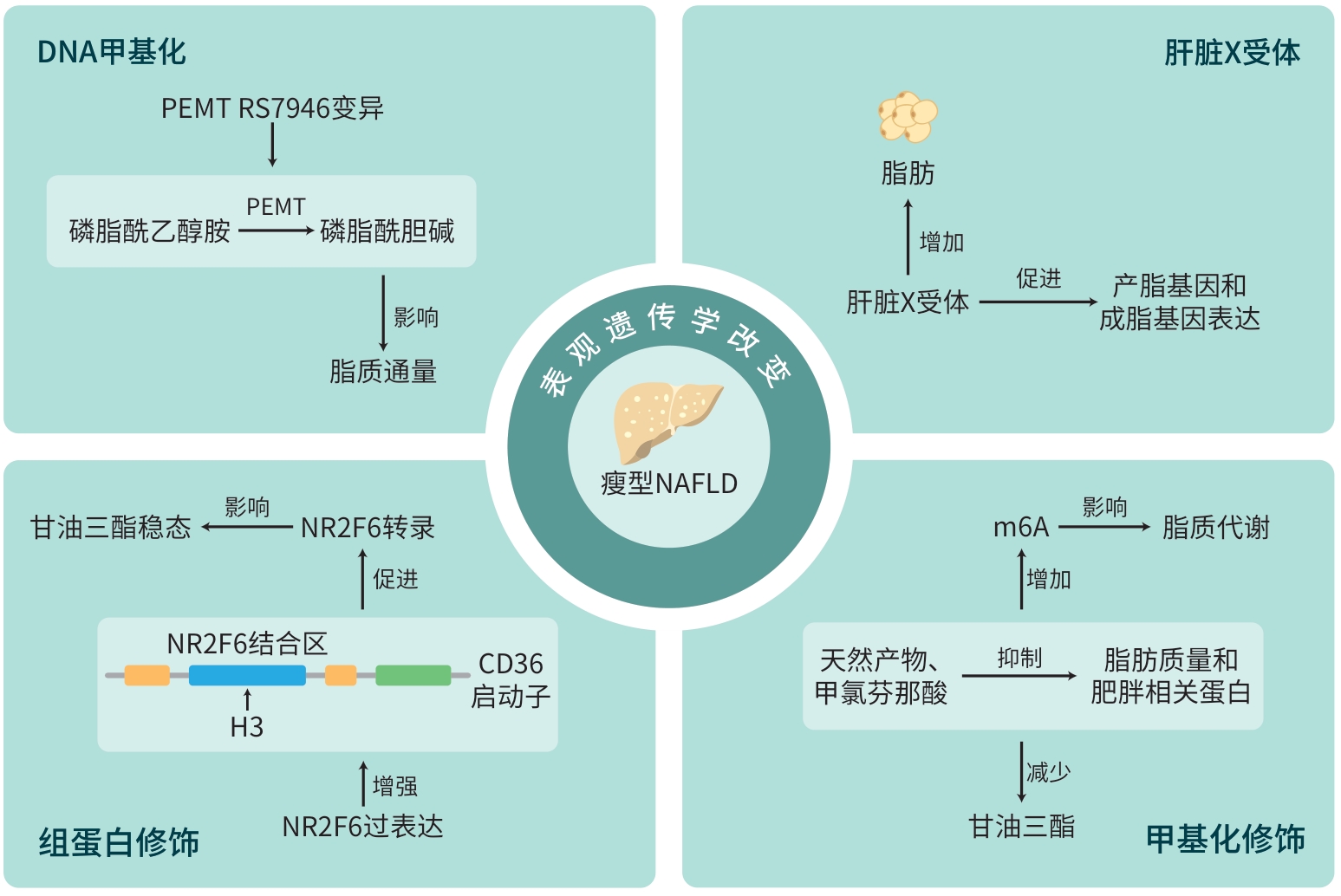
LI JY, XIN YG, LI JY, et al. Phosphatidylethanolamine N-methyltransferase: From functions to diseases[J]. Aging Dis, 2023, 14( 3): 879- 891. DOI: 10.14336/AD.2022.1025. |
| [30] |
BALE G, VISHNUBHOTLA RV, MITNALA S, et al. Whole-exome sequencing identifies a variant in phosphatidylethanolamine N-methyltransferase gene to be associated with lean-nonalcoholic fatty liver disease[J]. J Clin Exp Hepatol, 2019, 9( 5): 561- 568. DOI: 10.1016/j.jceh.2019.02.001. |
| [31] |
SEO JB, MOON HM, KIM WS, et al. Activated liver X receptors stimulate adipocyte differentiation through induction of peroxisome proliferator-activated receptor gamma expression[J]. Mol Cell Biol, 2004, 24( 8): 3430- 3444. DOI: 10.1128/MCB.24.8.3430-3444.2004. |
| [32] |
SHAMARDL HAMA, IBRAHIM NA, MERZEBAN DH, et al. Resveratrol and Dulaglutide ameliorate adiposity and liver dysfunction in rats with diet-induced metabolic syndrome: Role of SIRT-1/adipokines/PPARγ and IGF-1[J]. Daru, 2023, 31( 1): 13- 27. DOI: 10.1007/s40199-023-00458-y. |
| [33] |
ZHOU B, JIA LJ, ZHANG ZJ, et al. The nuclear orphan receptor NR2F6 promotes hepatic steatosis through upregulation of fatty acid transporter CD36[J]. Adv Sci(Weinh), 2020, 7( 21): 2002273. DOI: 10.1002/advs.202002273. |
| [34] |
CHEN MN, WONG CM. The emerging roles of N6-methyladenosine(m6A) deregulation in liver carcinogenesis[J]. Mol Cancer, 2020, 19( 1): 44. DOI: 10.1186/s12943-020-01172-y. |
| [35] |
HOU J, ZHANG H, LIU J, et al. YTHDF2 reduction fuels inflammation and vascular abnormalization in hepatocellular carcinoma[J]. Mol Cancer, 2019, 18( 1): 163. DOI: 10.1186/s12943-019-1082-3. |
| [36] |
HU Y, FENG Y, ZHANG LC, et al. GR-mediated FTO transactivation induces lipid accumulation in hepatocytes via demethylation of m 6A on lipogenic mRNAs[J]. RNA Biol, 2020, 17( 7): 930- 942. DOI: 10.1080/15476286.2020.1736868. |
| [37] |
PENG SM, XIAO W, JU DP, et al. Identification of entacapone as a chemical inhibitor of FTO mediating metabolic regulation through FOXO1[J]. Sci Transl Med, 2019, 11( 488): eaau7116. DOI: 10.1126/scitranslmed.aau7116. |
| [38] |
XU F, GUO WR. The progress of epigenetics in the development and progression of non-alcoholic fatty liver disease[J]. Liver Res, 2020, 4( 3): 118- 123. DOI: 10.1016/j.livres.2020.08.003. |
| [39] |
BOLLINGER E, PELOQUIN M, LIBERA J, et al. BDK inhibition acts as a catabolic switch to mimic fasting and improve metabolism in mice[J]. Mol Metab, 2022, 66: 101611. DOI: 10.1016/j.molmet.2022.101611. |
| [40] |
LEE S, WOO DC, KANG J, et al. The role of the histone methyltransferase EZH2 in liver inflammation and fibrosis in STAM NASH mice[J]. Biology(Basel), 2020, 9( 5): 93. DOI: 10.3390/biology9050093. |
| [41] |
XIN FZ, ZHAO ZH, LIU XL, et al. Escherichia fergusonii promotes nonobese nonalcoholic fatty liver disease by interfering with host hepatic lipid metabolism through its own msRNA 23487[J]. Cell Mol Gastroenterol Hepatol, 2022, 13( 3): 827- 841. DOI: 10.1016/j.jcmgh.2021.12.003. |
| [42] |
DONG H, WANG JJ, LI CM, et al. The phosphatidylethanolamine N-methyltransferase gene V175M single nucleotide polymorphism confers the susceptibility to NASH in Japanese population[J]. J Hepatol, 2007, 46( 5): 915- 920. DOI: 10.1016/j.jhep.2006.12.012. |
| [43] |
LIU CH, AMPUERO J, GIL-GÓMEZ A, et al. miRNAs in patients with non-alcoholic fatty liver disease: A systematic review and meta-analysis[J]. J Hepatol, 2018, 69( 6): 1335- 1348. DOI: 10.1016/j.jhep.2018.08.008. |
| [44] |
HUANG XY, YAO YC, HOU XL, et al. Macrophage SCAP contributes to metaflammation and lean NAFLD by activating STING-NF-κB signaling pathway[J]. Cell Mol Gastroenterol Hepatol, 2022, 14( 1): 1- 26. DOI: 10.1016/j.jcmgh.2022.03.006. |
| [45] |
PETTA S, CIMINNISI S, DI MARCO V, et al. Sarcopenia is associated with severe liver fibrosis in patients with non-alcoholic fatty liver disease[J]. Aliment Pharmacol Ther, 2017, 45( 4): 510- 518. DOI: 10.1111/apt.13889. |
| [46] |
HIMOTO T, MIYATAKE K, MAEBA T, et al. Verification of the nutritional and dietary factors associated with skeletal muscle index in Japanese patients with nonalcoholic fatty liver disease[J]. Can J Gastroenterol Hepatol, 2020, 2020: 3576974. DOI: 10.1155/2020/3576974. |
| [47] |
NAIR VD, GE YC, LI SD, et al. Sedentary and trained older men have distinct circulating exosomal microRNA profiles at baseline and in response to acute exercise[J]. Front Physiol, 2020, 11: 605. DOI: 10.3389/fphys.2020.00605. |







 DownLoad:
DownLoad: