| [1] |
|
| [2] |
HENNEDIGE TP, NEO WT, VENKATESH SK. Imaging of malignancies of the biliary tract- an update[J]. Cancer Imag, 2014, 14( 1): 14. DOI: 10.1186/1470-7330-14-14. |
| [3] |
BANALES JM, MARIN JJG, LAMARCA A, et al. Cholangiocarcinoma 2020: The next horizon in mechanisms and management[J]. Nat Rev Gastroenterol Hepatol, 2020, 17( 9): 557- 588. DOI: 10.1038/s41575-020-0310-z. |
| [4] |
VOGEL A, BRIDGEWATER J, EDELINE J, et al. Biliary tract cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up[J]. Ann Oncol, 2023, 34( 2): 127- 140. DOI: 10.1016/j.annonc.2022.10.506. |
| [5] |
FLORIO AA, FERLAY J, ZNAOR A, et al. Global trends in intrahepatic and extrahepatic cholangiocarcinoma incidence from 1993 to 2012[J]. Cancer, 2020, 126( 11): 2666- 2678. DOI: 10.1002/cncr.32803. |
| [6] |
KHAN SA, TOLEDANO MB, TAYLOR-ROBINSON SD. Epidemiology, risk factors, and pathogenesis of cholangiocarcinoma[J]. Hpb, 2008, 10( 2): 77- 82. DOI: 10.1080/13651820801992641. |
| [7] |
RANDI G, MALVEZZI M, LEVI F, et al. Epidemiology of biliary tract cancers: An update[J]. Ann Oncol, 2009, 20( 1): 146- 159. DOI: 10.1093/annonc/mdn533. |
| [8] |
KHAN SA, TAVOLARI S, BRANDI G. Cholangiocarcinoma: Epidemiology and risk factors[J]. Liver Int, 2019, 39( S1): 19- 31. DOI: 10.1111/liv.14095. |
| [9] |
LEE SS, KIM MH, LEE SK, et al. Clinicopathologic review of 58 patients with biliary papillomatosis[J]. Cancer, 2004, 100( 4): 783- 793. DOI: 10.1002/cncr.20031. |
| [10] |
LAMARCA A, EDELINE J, MCNAMARA MG, et al. Current standards and future perspectives in adjuvant treatment for biliary tract cancers[J]. Cancer Treat Rev, 2020, 84: 101936. DOI: 10.1016/j.ctrv.2019.101936. |
| [11] |
JAVLE M, LEE S, AZAD NS, et al. Temporal changes in cholangiocarcinoma incidence and mortality in the United States from 2001 to 2017[J]. Oncologist, 2022, 27( 10): 874- 883. DOI: 10.1093/oncolo/oyac150. |
| [12] |
IZQUIERDO-SANCHEZ L, LAMARCA A, LA CASTA A, et al. Cholangiocarcinoma landscape in Europe: Diagnostic, prognostic and therapeutic insights from the ENSCCA Registry[J]. J Hepatol, 2022, 76( 5): 1109- 1121. DOI: 10.1016/j.jhep.2021.12.010. |
| [13] |
LI B, LI ZS, QIU ZQ, et al. Surgical treatment of hilar cholangiocarcinoma: Retrospective analysis[J]. BJS Open, 2023, 7( 3): zrad024. DOI: 10.1093/bjsopen/zrad024. |
| [14] |
LIU Q, JIANG N, TIAN EY, et al. Short-term outcomes of robotic versus open pancreaticoduodenectomy in elderly patients: A multicenter retrospective cohort study[J]. Int J Surg, 2022, 104: 106819. DOI: 10.1016/j.ijsu.2022.106819. |
| [15] |
KABIR T, TAN HL, SYN NL, et al. Outcomes of laparoscopic, robotic, and open pancreatoduodenectomy: A network meta-analysis of randomized controlled trials and propensity-score matched studies[J]. Surgery, 2022, 171( 2): 476- 489. DOI: 10.1016/j.surg.2021.07.020. |
| [16] |
ZUREIKAT AH, POSTLEWAIT LM, LIU Y, et al. A multi-institutional comparison of perioperative outcomes of robotic and open pancreaticoduodenectomy[J]. Ann Surg, 2016, 264( 4): 640- 649. DOI: 10.1097/sla.0000000000001869. |
| [17] |
HEIMBACH J, GORES G, HADDOCK M, et al. Liver transplantation for unresectable perihilar cholangiocarcinoma[J]. Semin Liver Dis, 2004, 24( 2): 201- 207. DOI: 10.1055/s-2004-828896. |
| [18] |
VALLE J, WASAN H, PALMER DH, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer[J]. N Engl J Med, 2010, 362( 14): 1273- 1281. DOI: 10.1056/nejmoa0908721. |
| [19] |
KIM ST, KANG JH, LEE J, et al. Capecitabine plus oxaliplatin versus gemcitabine plus oxaliplatin as first-line therapy for advanced biliary tract cancers: A multicenter, open-label, randomized, phase III, noninferiority trial[J]. Ann Oncol, 2019, 30( 5): 788- 795. DOI: 10.1093/annonc/mdz058. |
| [20] |
LAMARCA A, PALMER DH, WASAN HS, et al. Second-line FOLFOX chemotherapy versus active symptom control for advanced biliary tract cancer(ABC-06): A phase 3, open-label, randomised, controlled trial[J]. Lancet Oncol, 2021, 22( 5): 690- 701. DOI: 10.1016/s1470-2045(21)00027-9. |
| [21] |
HYUNG J, KIM I, KIM KP, et al. Treatment with liposomal irinotecan plus fluorouracil and leucovorin for patients with previously treated metastatic biliary tract cancer: The phase 2b NIFTY randomized clinical trial[J]. JAMA Oncol, 2023, 9( 5): 692. DOI: 10.1001/jamaoncol.2023.0016. |
| [22] |
YANG JF, WANG J, ZHOU HB, et al. Efficacy and safety of endoscopic radiofrequency ablation for unresectable extrahepatic cholangiocarcinoma: A randomized trial[J]. Endoscopy, 2018, 50( 8): 751- 760. DOI: 10.1055/s-0043-124870. |
| [23] |
GAO DJ, YANG JF, MA SR, et al. Endoscopic radiofrequency ablation plus plastic stent placement versus stent placement alone for unresectable extrahepatic biliary cancer: A multicenter randomized controlled trial[J]. Gastrointest Endosc, 2021, 94( 1): 91- 100.e2. DOI: 10.1016/j.gie.2020.12.016. |
| [24] |
ORTNER MEJ, LIEBETRUTH J, SCHREIBER S, et al. Photodynamic therapy of nonresectable cholangiocarcinoma[J]. Gastroenterology, 1998, 114( 3): 536- 542. DOI: 10.1016/s0016-5085(98)70537-2. |
| [25] |
ZOEPF T, JAKOBS R, ARNOLD JC, et al. Palliation of nonresectable bile duct cancer: Improved survival after photodynamic therapy[J]. Am J Gastroenterology, 2005, 100( 11): 2426- 2430. DOI: 10.1111/j.1572-0241.2005.00318.x. |
| [26] |
NANASHIMA A, YAMAGUCHI H, SHIBASAKI S, et al. Adjuvant photodynamic therapy for bile duct carcinoma after surgery: A preliminary study[J]. J Gastroenterol, 2004, 39( 11): 1095- 1101. DOI: 10.1007/s00535-004-1449-z. |
| [27] |
LI ZY, JIANG XF, XIAO H, et al. Long-term results of ERCP- or PTCS-directed photodynamic therapy for unresectable hilar cholangiocarcinoma[J]. Surg Endosc, 2021, 35( 10): 5655- 5664. DOI: 10.1007/s00464-020-08095-1. |
| [28] |
WIEDMANN M, CACA K, BERR F, et al. Neoadjuvant photodynamic therapy as a new approach to treating hilar cholangiocarcinoma: A Phase II pilot study[J]. Cancer, 2003, 97( 11): 2783- 2790. DOI: 10.1002/cncr.11401. |
| [29] |
GUPTA P, MARALAKUNTE M, SAGAR S, et al. Efficacy and safety of irreversible electroporation for malignant liver tumors: A systematic review and meta-analysis[J]. Eur Radiol, 2021, 31( 9): 6511- 6521. DOI: 10.1007/s00330-021-07742-y. |
| [30] |
FRANZESE C, BONU ML, COMITO T, et al. Stereotactic body radiotherapy in the management of oligometastatic and recurrent biliary tract cancer: Single-institution analysis of outcome and toxicity[J]. J Cancer Res Clin Oncol, 2020, 146( 9): 2289- 2297. DOI: 10.1007/s00432-020-03285-9. |
| [31] |
MENG SY, DING GC, SHI CL, et al. Observation of the efficacy of stereotactic radiotherapy combined with chemotherapy for locally advanced perihilar cholangiocarcinoma[J]. Acad J Chinese PLA Postgrad Med Sch, 2019, 40( 11): 1014- 1017, 1033. DOI: 10.3969/j.issn.2095-5227.2019.11.002. |
| [32] |
MELLMAN I, COUKOS G, DRANOFF G. Cancer immunotherapy comes of age[J]. Nature, 2011, 480( 7378): 480- 489. DOI: 10.1038/nature10673. |
| [33] |
POSTOW MA, CALLAHAN MK, BARKER CA, et al. Immunologic correlates of the abscopal effect in a patient with melanoma[J]. N Engl J Med, 2012, 366( 10): 925- 931. DOI: 10.1056/nejmoa1112824. |
| [34] |
YOON SB, WOO SM, CHUN JW, et al. The predictive value of PD-L1 expression in response to anti-PD-1/PD-L1 therapy for biliary tract cancer: A systematic review and meta-analysis[J]. Front Immunol, 2024, 15: 1321813. DOI: 10.3389/fimmu.2024.1321813. |
| [35] |
TOMLINSON JL, VALLE JW, ILYAS SI. Immunobiology of cholangiocarcinoma[J]. J Hepatol, 2023, 79( 3): 867- 875. DOI: 10.1016/j.jhep.2023.05.010. |
| [36] |
PIHA-PAUL SA, OH DY, UENO M, et al. Efficacy and safety of pembrolizumab for the treatment of advanced biliary cancer: Results from the KEYNOTE-158 and KEYNOTE-028 studies[J]. Int J Cancer, 2020, 147( 8): 2190- 2198. DOI: 10.1002/ijc.33013. |
| [37] |
KIM RD, CHUNG V, ALESE OB, et al. A phase 2 multi-institutional study of nivolumab for patients with advanced refractory biliary tract cancer[J]. JAMA Oncol, 2020, 6( 6): 888. DOI: 10.1001/jamaoncol.2020.0930. |
| [38] |
ABOU-ALFA GK, SAHAI V, HOLLEBECQUE A, et al. Pemigatinib for previously treated, locally advanced or metastatic cholangiocarcinoma: A multicentre, open-label, phase 2 study[J]. Lancet Oncol, 2020, 21( 5): 671- 684. DOI: 10.1016/s1470-2045(20)30109-1. |
| [39] |
OHBA A, MORIZANE C, KAWAMOTO Y, et al. Trastuzumab deruxtecan in human epidermal growth factor receptor 2-expressing biliary tract cancer(HERB; NCCH1805): A multicenter, single-arm, phase Ⅱ trial[J]. J Clin Oncol, 2024, 42( 27): 3207- 3217. DOI: 10.1200/jco.23.02010. |
| [40] |
JAVLE M, BORAD MJ, AZAD NS, et al. Pertuzumab and trastuzumab for HER2-positive, metastatic biliary tract cancer(MyPathway): A multicentre, open-label, phase 2a, multiple basket study[J]. Lancet Oncol, 2021, 22( 9): 1290- 1300. DOI: 10.1016/s1470-2045(21)00336-3. |
| [41] |
HARDING JJ, FAN J, OH DY, et al. Zanidatamab for HER2-amplified, unresectable, locally advanced or metastatic biliary tract cancer(HERIZON-BTC-01): A multicentre, single-arm, phase 2b study[J]. Lancet Oncol, 2023, 24( 7): 772- 782. DOI: 10.1016/s1470-2045(23)00242-5. |
| [42] |
OH DY, RUTH HE A, QIN SK, et al. Durvalumab plus gemcitabine and cisplatin in advanced biliary tract cancer[J]. NEJM Evid, 2022, 1( 8). DOI: 10.1056/evidoa2200015. |
| [43] |
KELLEY RK, UENO M, YOO C, et al. Pembrolizumab in combination with gemcitabine and cisplatin compared with gemcitabine and cisplatin alone for patients with advanced biliary tract cancer(KEYNOTE-966): A randomised, double-blind, placebo-controlled, phase 3 trial[J]. Lancet, 2023, 401( 10391): 1853- 1865. DOI: 10.1016/s0140-6736(23)00727-4. |
| [44] |
SHI GM, HUANG XY, MA L, et al. First-line tislelizumab and ociperlimab combined with gemcitabine and cisplatin in advanced biliary tract cancer(ZSAB-TOP): A multicenter, single-arm, phase 2 study[J]. Sig Transduct Target Ther, 2025, 10: 260. DOI: 10.1038/s41392-025-02356-y. |
| [45] |
SHI GM, HUANG XY, WU D, et al. Toripalimab combined with lenvatinib and GEMOX is a promising regimen as first-line treatment for advanced intrahepatic cholangiocarcinoma: A single-center, single-arm, phase 2 study[J]. Sig Transduct Target Ther, 2023, 8: 106. DOI: 10.1038/s41392-023-01317-7. |



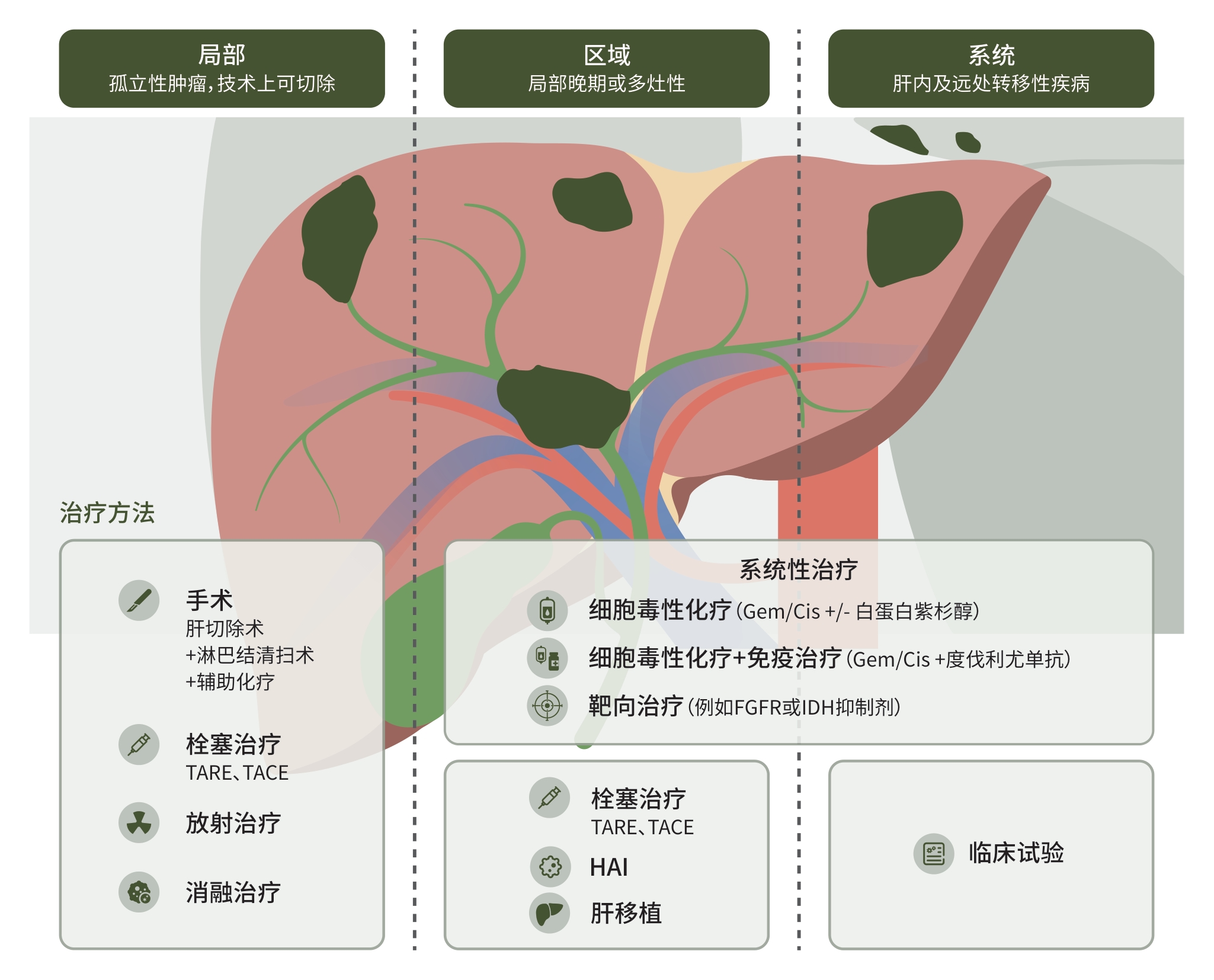




 DownLoad:
DownLoad: