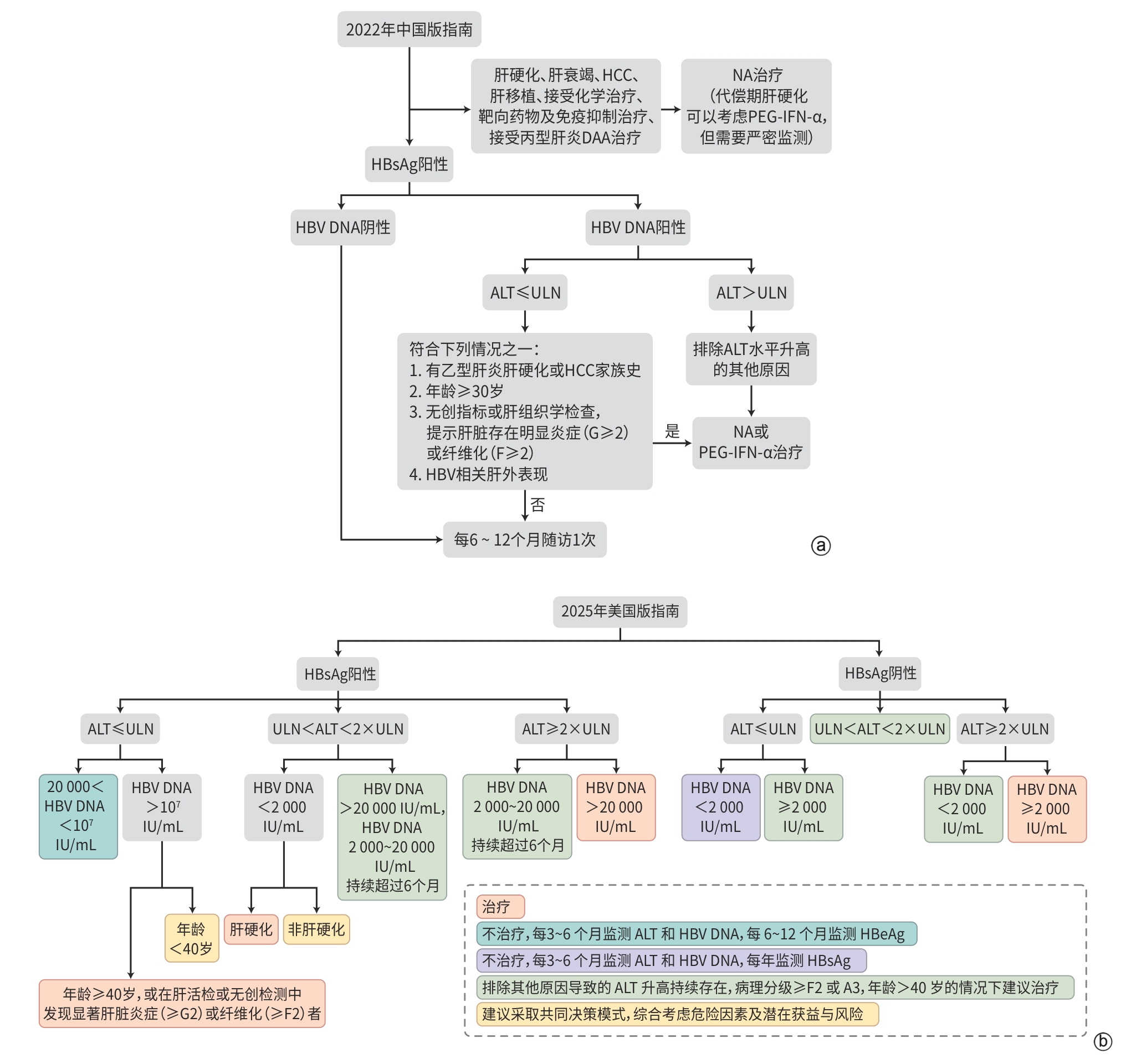
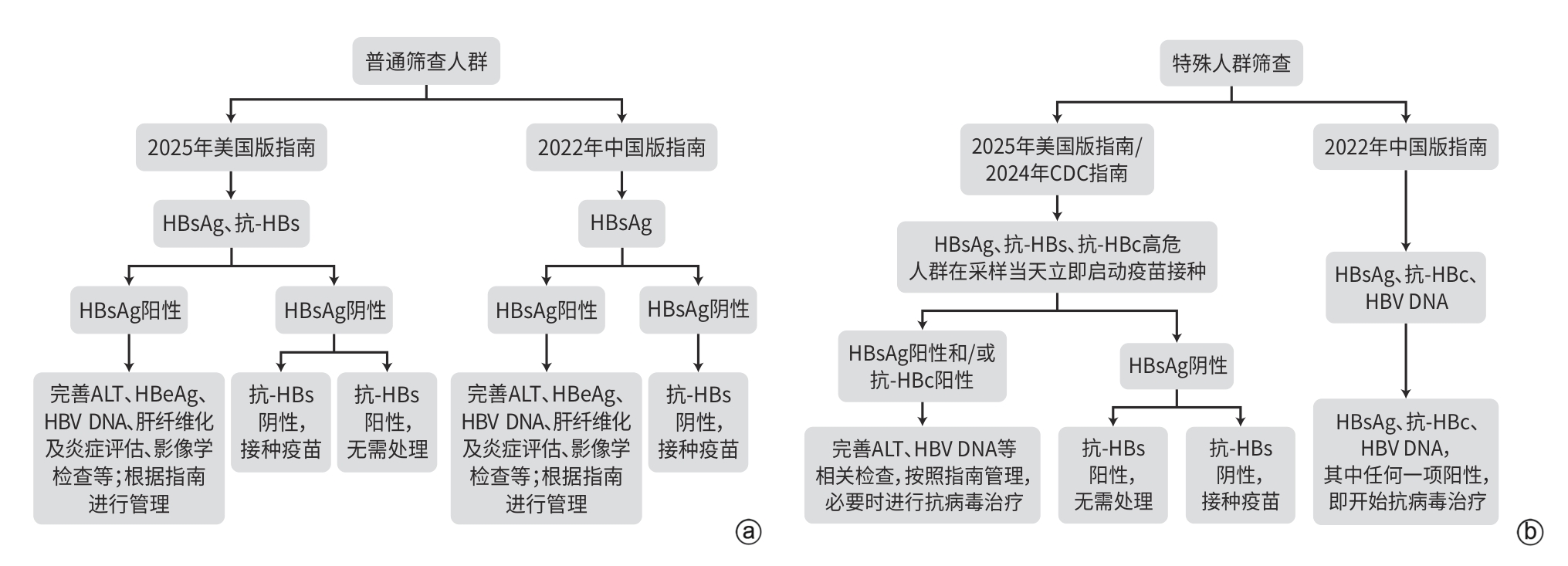
This article provides a systematic comparative analysis of the key recommendations between the “AASLD/IDSA Practice Guidelines for the Treatment of Chronic Hepatitis B (2025 edition)” and the “Chinese Guidelines for the Prevention and Treatment of Chronic Hepatitis B (2022 edition)”. The comparison focuses on the following seven core domains: background and significance; methodology; prevention and screening strategies; treatment indications and indicators for treatment discontinuation; drug selection and therapeutic strategies; management of special populations; and liver cancer screening and management. By examining areas of consensus and divergence, this article highlights the differences and similarities in evidence-based approaches and clinical practice pathways between the two guidelines, in order to provide a reference for clinical decision-making concerning individualized diagnosis and treatment, as well as targeted management of hepatitis B patients in clinical practice.







 DownLoad:
DownLoad: