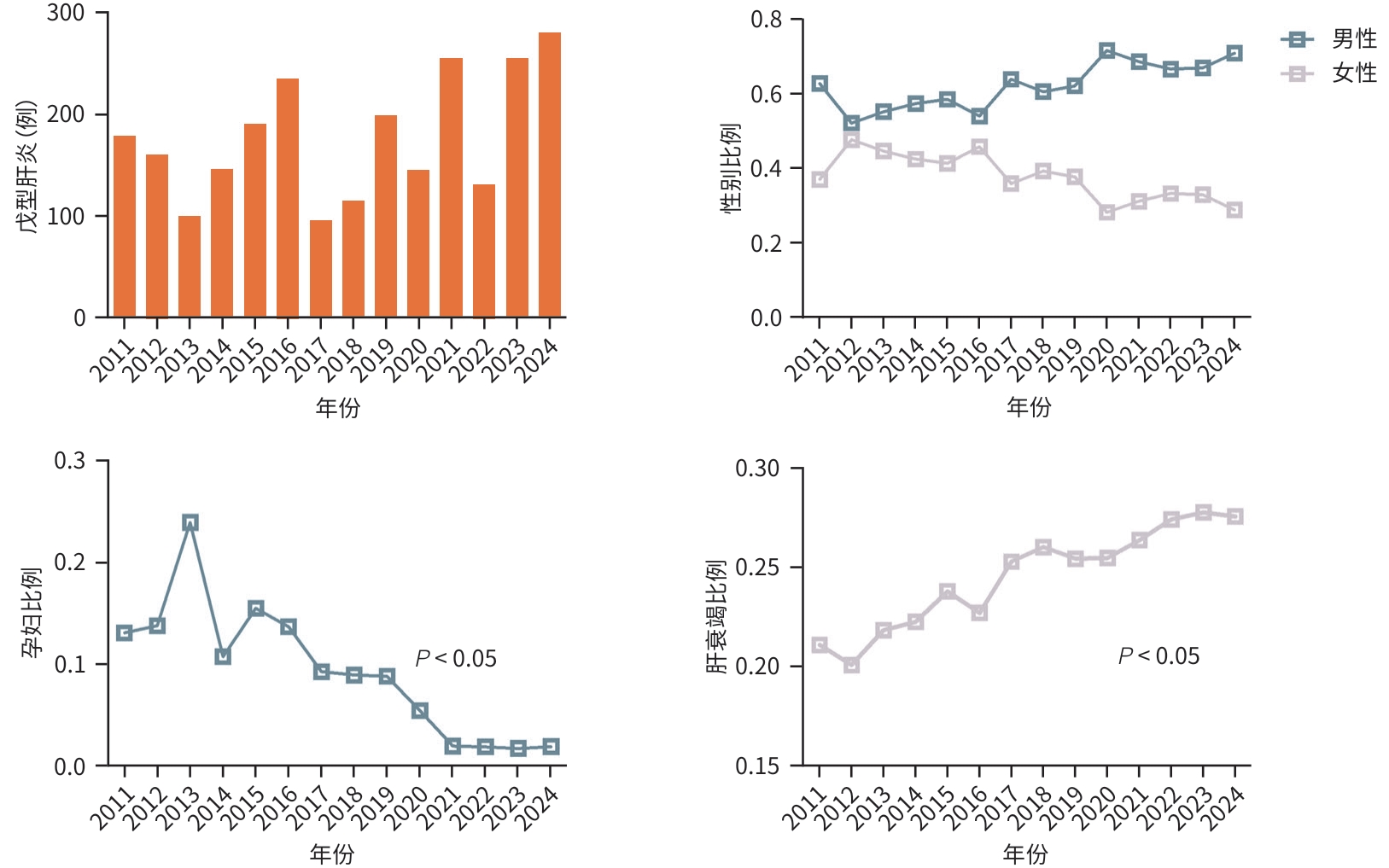
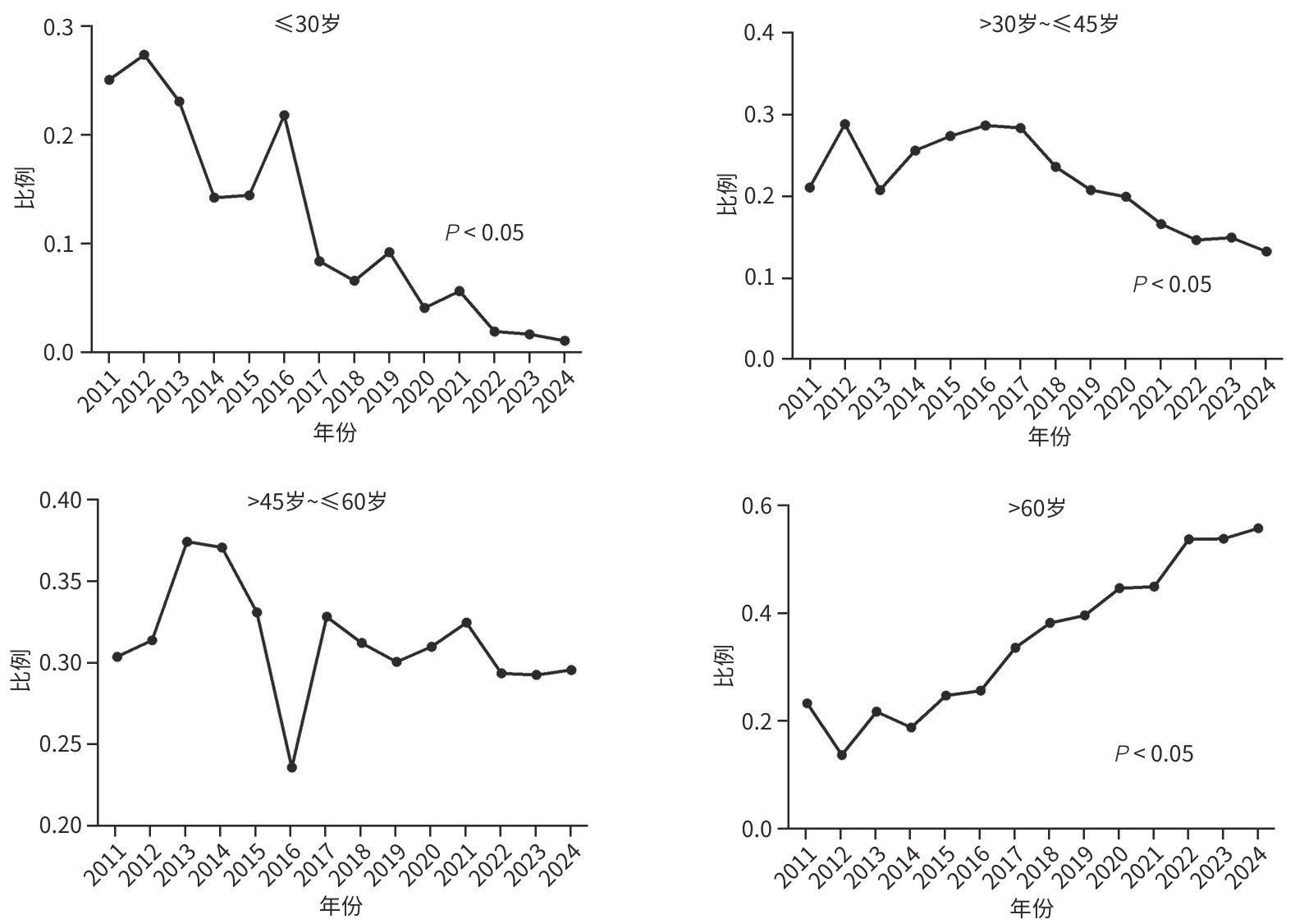
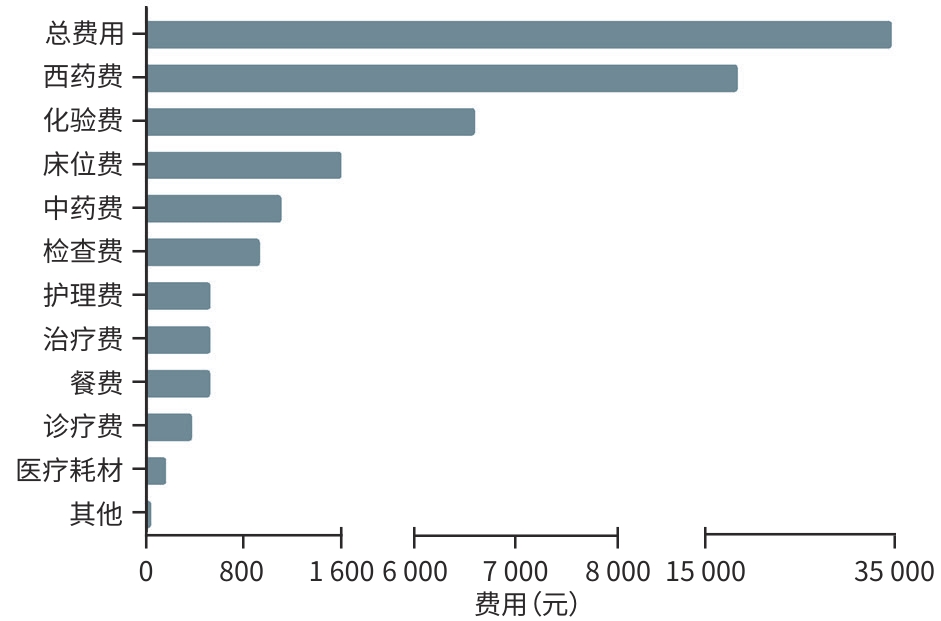
| [1] |
SONGTANIN B, MOLEHIN AJ, BRITTAN K, et al. Hepatitis E virus infections: Epidemiology, genetic diversity, and clinical considerations[J]. Viruses, 2023, 15( 6): 1389. DOI: 10.3390/v15061389. |
| [2] |
|
| [3] |
ASLAN AT, BALABAN HY. Hepatitis E virus: Epidemiology, diagnosis, clinical manifestations, and treatment[J]. World J Gastroenterol, 2020, 26( 37): 5543- 5560. DOI: 10.3748/wjg.v26.i37.5543. |
| [4] |
DONG R, CHANG DC, LUO ZH, et al. The burden of HEV-related acute liver failure in Bangladesh, China and India: A systematic review and meta-analysis[J]. BMC Public Health, 2023, 23( 1): 2369. DOI: 10.1186/s12889-023-17302-2. |
| [5] |
CHEN C, WANG ML, LI WX, et al. Hepatitis e virus infection increases the risk of obstetric complications and perinatal adverse outcomes in pregnant women with chronic hepatitis B virus infection[J]. Eur Rev Med Pharmacol Sci, 2024, 28( 5): 1904- 1912. DOI: 10.26355/eurrev_202403_35604. |
| [6] |
ZHANG S, CHEN C, PENG J, et al. Investigation of underlying comorbidities as risk factors for symptomatic human hepatitis E virus infection[J]. Aliment Pharmacol Ther, 2017, 45( 5): 701- 713. DOI: 10.1111/apt.13938. |
| [7] |
CHEN C, ZHANG SY, ZHANG DD, et al. Clinical features of acute hepatitis E super-infections on chronic hepatitis B[J]. World J Gastroenterol, 2016, 22( 47): 10388. DOI: 10.3748/wjg.v22.i47.10388. |
| [8] |
LI Q, CHEN C, HUANG CL, et al. Noninvasive models for predicting poor prognosis of chronic HBV infection patients precipitating acute HEV infection[J]. Sci Rep, 2020, 10( 1): 2753. DOI: 10.1038/s41598-020-59670-4. |
| [9] |
BUTI M, RUIZ-COBO JC, ESTEBAN R, et al. Hepatitis E as a trigger for acute-on-chronic liver failure[J]. Clin Mol Hepatol, 2025, 31: S196- S204. DOI: 10.3350/cmh.2024.0758. |
| [10] |
DUAN BF, FENG Y. Current knowledge on the epidemiology and detection methods of hepatitis E virus in China[J]. Virol J, 2024, 21( 1): 307. DOI: 10.1186/s12985-024-02576-8. |
| [11] |
HE ZW, LIU DY, LIU BL, et al. Prevalence of hepatitis E virus in swine in China: A systematic review with meta-analysis(2004-2023)[J]. Front Vet Sci, 2024, 11: 1472658. DOI: 10.3389/fvets.2024.1472658. |
| [12] |
Chinese Society of Hepatology, Chinese Medical Association. Consensus on prevention and treatment of hepatitis E[J]. Chin J Hepatol, 2022, 30( 8): 820- 831. DOI: 10.3760/cma.j.cn501113-20220729-00401. |
| [13] |
People’s Hospital of Peking University, Institute of Hepatology of Peking University, National Institute for the Control of Pharmaceutical and Biological Products, et al. Diagnostic Criteria for Hepatitis E[S]. 2008.
北京大学人民医院, 北京大学肝病研究所, 中国药品生物制品检定所, 等. 戊型病毒性肝炎诊断标准[S]. 2008.
|
| [14] |
XU B. Clinical features and risk factors of acute hepatitis E with severe jaundice[J]. World J Gastroenterol, 2012, 18( 48): 7279. DOI: 10.3748/wjg.v18.i48.7279. |
| [15] |
|
| [16] |
The Subgroup of Severe Liver Disease and Artificial Liver in the Hepatology Branch of the Chinese Medical Association, and the Subgroup of Liver Failure and Artificial Liver in the Infectious Diseases Branch of the Chinese Medical Association. Guidelines for the diagnosis and treatment of liver failure(2024 Edition)[J]. J Clin Hepatol, 2024, 40( 12): 2371- 2387. DOI: 10.12449/JCH241206. 中华医学会感染病学分会肝衰竭与人工肝学组, 中华医学会肝病学分会重型肝病与人工肝学组. 肝衰竭诊治指南(2024年版)[J]. 临床肝胆病杂志, 2024, 40( 12): 2371- 2387. DOI: 10.12449/JCH241206. |
| [17] |
LIU TX, LI J, YIN X, et al. Establishment of enterically transmitted hepatitis virus animal models using lipid nanoparticle-based full-length viral genome RNA delivery system[J]. Gut, 2025, 74( 3): 467- 476. DOI: 10.1136/gutjnl-2024-332784. |
| [18] |
WASUWANICH P, WEN TS, EGERMAN RS, et al. Epidemiology and outcomes of hepatitis E virus-associated hospitalisations in the United States with a focus on pregnancy: A nationwide population study, 1998-2020[J]. J Viral Hepat, 2024, 31( 11): 710- 719. DOI: 10.1111/jvh.13994. |
| [19] |
KHONGVIWATSATHIEN S, THAWEERAT W, ATTHAKITMONGKOL T, et al. A comparison of clinical manifestations and outcomes between acute sporadic hepatitis a and hepatitis E infections in Thailand[J]. Viruses, 2023, 15( 9): 1888. DOI: 10.3390/v15091888. |
| [20] |
LI XY, ZHOU Y, HUANG H, et al. Progress in disease burden researches[J]. Chin J Public Health, 2018, 34( 5): 777- 780. DOI: 10.11847/zgggws1118319. |
| [21] |
LI YC. New progress in detection methods of viral hepatitis serum markers[J]. J Mol Diagn Ther, 2020, 12( 12): 1753- 1756.
李轶春. 病毒性肝炎血清标志物检测方法新进展[J]. 分子诊断与治疗杂志, 2020, 12( 12): 1753- 1756.
|
| [22] |
Chinese Consortium for the Study of Hepatitis E(CCSHE), Chinese Physician Association for Infectious Disease, National Clinical Research Center for Infectious Diseases. Expert consensus on the process of in-hospital screening and management of viral hepatitis E in China(2023)[J]. J Clin Hepatol, 2023, 39( 4): 785- 794. DOI: 10.3969/j.issn.1001-5256.2023.04.008. |
| [23] |
GENG YS, SHI TF, WANG YC. Epidemiology of hepatitis E[M]// Hepatitis E Virus. Singapore: Springer Nature Singapore, 2023: 33- 48. DOI: 10.1007/978-981-99-1304-6_3. |
| [24] |
HORVATITS T, SCHULZE ZUR WIESCH J, LÜTGEHETMANN M, et al. The clinical perspective on hepatitis E[J]. Viruses, 2019, 11( 7): 617. DOI: 10.3390/v11070617. |
| [25] |
TOSONE G, SIMEONE D, SPERA AM, et al. Epidemiology and pathogenesis of fulminant viral hepatitis in pregnant women[J]. Minerva Obstet Gynecol, 2018, 70( 4): 480- 486. DOI: 10.23736/s0026-4784.17.04107-7. |
| [26] |
|
| [27] |
WANG YY, HUANG J, LAN Y, et al. Investigation and analysis on the awareness rate of hepatitis E prevention knowledge and vaccination willingness of employees in Nanning[J]. Appl Prev Med, 2025, 31( 1): 91- 95.
王芸芸, 黄菊, 蓝莹, 等. 南宁市从业人员戊型肝炎防治知识知晓率及疫苗接种意愿调查分析[J]. 应用预防医学, 2025, 31( 1): 91- 95.
|
| [28] |
CHEN Q. Analysis on detection of Hepatitis A and E and influencing factors of"knowledge, attitude and behavior" in some practitioners in a district of Hangzhou, 2015-2016[J]. Chin J Public Health Manag, 2018, 34( 2): 271- 273. DOI: 10.19568/j.cnki.23-1318.2018.02.037. |
| [29] |
ZHU YP, ZHU CW. Advances in the prevention and treatment of the high-risk population for hepatitis E[J]. J Clin Hepatol, 2023, 39( 11): 2524- 2529. DOI: 10.3969/j.issn.1001-5256.2023.11.002. |
| [30] |
CHIBBER RM, USMANI MA, AL-SIBAI MH. Should HEV infected mothers breast feed?[J]. Arch Gynecol Obstet, 2004, 270( 1): 15- 20. DOI: 10.1007/s00404-002-0466-5. |
| [31] |
ZHONG GH, ZHUANG CL, HU XW, et al. Safety of hepatitis E vaccination for pregnancy: A post-hoc analysis of a randomized, double-blind, controlled phase 3 clinical trial[J]. Emerg Microbes Infect, 2023, 12( 1): 2185456. DOI: 10.1080/22221751.2023.2185456. |



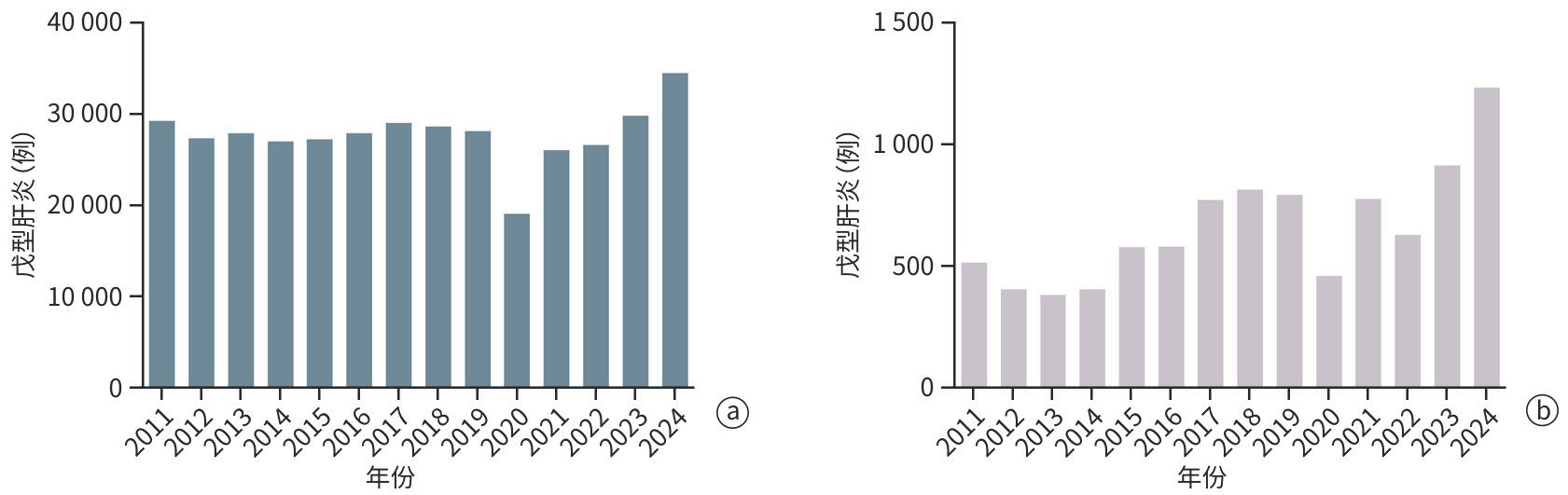




 DownLoad:
DownLoad: