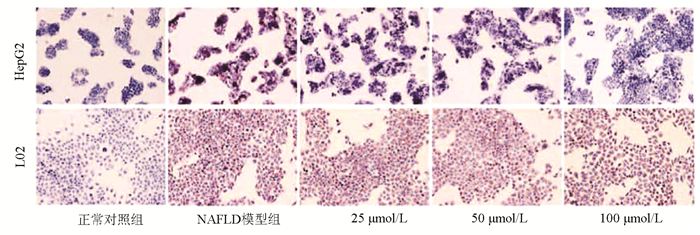
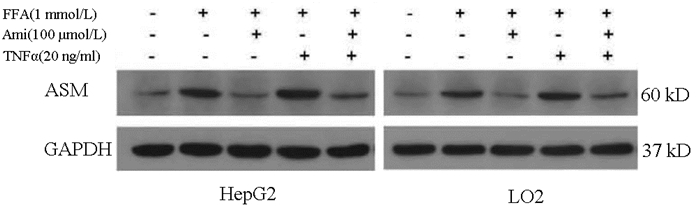
| [1] |
MUNDI MS, VELAPATI S, PATEL J, et al. Evolution of NAFLD and its management[J]. Nutr Clin Pract, 2020, 35(1): 72-84. DOI: 10.1002/ncp.10449 |
| [2] |
JIANG YZ, NIE HM, WANG R. Research advances in the pathogenesis of nonalcoholic fatty liver disease[J]. J Clin Hepatol, 2019, 35(11): 2588-2591. (in Chinese) DOI: 10.3969/j.issn.1001-5256.2019.11.044 |
| [3] |
|
| [4] |
YANG L, JIN GH, ZHOU JY. The role of ceramide in the pathogenesis of alcoholic liver disease[J]. Alcohol Alcohol, 2016, 51(3): 251-257. DOI: 10.1093/alcalc/agv119 |
| [5] |
RÉGNIER M, POLIZZI A, GUILLOU H, et al. Sphingolipid metabolism in non-alcoholic fatty liver diseases[J]. Biochimie, 2019, 159: 9-22. DOI: 10.1016/j.biochi.2018.07.021 |
| [6] |
UNGER RH. Lipotoxic diseases[J]. Annu Rev Med, 2002, 53(4): 319-336.
|
| [7] |
MALDONADO-HERNÁNDEZ J, SALDAÑA-DÁVILA GE, PIÑA-AGUERO MI, et al. Association between plasmatic ceramides profile and AST/ALT Ratio: C14:0 ceramide as predictor of hepatic steatosis in adolescents independently of obesity[J]. Can J Gastroenterol Hepatol, 2017, 2017: 3689375.
|
| [8] |
APOSTOLOPOULOU M, GORDILLO R, KOLIAKI C, et al. Specific hepatic sphingolipids relate to insulin resistance, oxidative stress, and inflammation in nonalcoholic steatohepatitis[J]. Diabetes Care, 2018, 41(6): 1235-1243. DOI: 10.2337/dc17-1318 |
| [9] |
LUUKKONEN PK, ZHOU Y, SÄDEVIRTA S, et al. Hepatic ceramides dissociate steatosis and insulin resistance in patients with non-alcoholic fatty liver disease[J]. J Hepatol, 2016, 64(5): 1167-1175. DOI: 10.1016/j.jhep.2016.01.002 |
| [10] |
|
| [11] |
FERNANDEZ A, MATIAS N, FUCHO R, et al. ASMase is required for chronic alcohol induced hepatic endoplasmic reticulum stress and mitochondrial cholesterol loading[J]. J Hepatol, 2013, 59(4): 805-813. DOI: 10.1016/j.jhep.2013.05.023 |
| [12] |
FUCHO R, MARTÍNEZ L, BAULIES A, et al. ASMase regulates autophagy and lysosomal membrane permeabilization and its inhibition prevents early stage non-alcoholic steatohepatitis[J]. J Hepatol, 2014, 61(5): 1126-1134. DOI: 10.1016/j.jhep.2014.06.009 |
| [13] |
LIANGPUNSAKUL S, RAHMINI Y, ROSS RA, et al. Imipramine blocks ethanol-induced ASMase activation, ceramide generation, and PP2A activation, and ameliorates hepatic steatosis in ethanol-fed mice[J]. Am J Physiol Gastrointest Liver Physiol, 2012, 302(5): g515-g523. DOI: 10.1152/ajpgi.00455.2011 |
| [14] |
GARCIA-RUIZ C, MATO JM, VANCE D, et al. Acid sphingomyelinase-ceramide system in steatohepatitis: A novel target regulating multiple pathways[J]. J Hepatol, 2015, 62(1): 219-233. DOI: 10.1016/j.jhep.2014.09.023 |
| [15] |
MVHLE C, WEINLAND C, GULBINS E, et al. Peripheral acid sphingomyelinase activity is associated with biomarkers and phenotypes of alcohol use and dependence in patients and healthy controls[J]. Int J Mol Sci, 2018, 19(12): 4028. DOI: 10.3390/ijms19124028 |
| [16] |
GUAN Y, LI X, UMETANI M, et al. Tricyclic antidepressant amitriptyline inhibits autophagic flux and prevents tube formation in vascular endothelial cells[J]. Basic Clin Pharmacol Toxicol, 2019, 124(4): 370-384. DOI: 10.1111/bcpt.13146 |
| [17] |
LU Z, LI Y, SYN WK, et al. Amitriptyline inhibits nonalcoholic steatohepatitis and atherosclerosis induced by high-fat diet and LPS through modulation of sphingolipid metabolism[J]. Am J Physiol Endocrinol Metab, 2020, 318(2): e131-e144. DOI: 10.1152/ajpendo.00181.2019 |










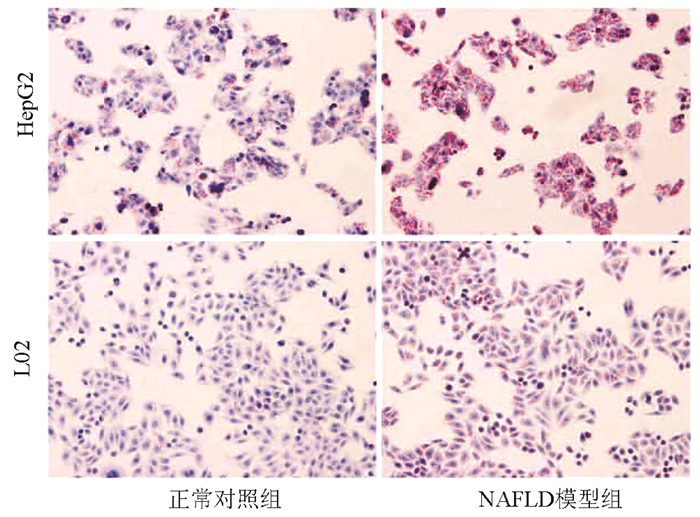




 DownLoad:
DownLoad: