| [1] |
|
| [2] |
Chinese Foundation for Hepatitis Prevention and Control; Chinese Society of Infectious Diseases, Chinese Medical Association; Chinese Society of Hepatology, Chinese Medical Association. Management algorithm for interrupting mother-to-child transmission of hepatitis B[J]. J Clin Hepatol, 2017, 33(7): 1214-1217. (in Chinese) DOI: 10.3969/j.issn.1001-5256.2017.07.003 |
| [3] |
HOU J, CUI F, DING Y, et al. Management algorithm for interrupting mother-to-child transmission of hepatitis B virus[J]. Clin Gastroenterol Hepatol, 2019, 17(10): 1929-1936. e1. DOI: 10.1016/j.cgh.2018.10.007 |
| [4] |
Chinese Society of Infectious Diseases, Chinese Medical Association; Chinese Society of Hepatology, Chinese Medical Association. Guidelines for the prevention and treatment of chronic hepatitis B (version 2019)[J]. J Clin Hepatol, 2019, 35(12): 2648-2669. (in Chinese) DOI: 10.3969/j.issn.1001-5256.2019.12.007 |
| [5] |
Obstetrics Subgroup, Chinese Society of Obstetrics and Gynecology, Chinese Medical Association; Chinese Society of Perinatal Medicine, Chinese Medical Association. 2020 clinical guidelines on prevention of mother-to-child transmission of hepatitis B virus[J]. J Clin Hepatol, 2020, 36(7): 1474-1481. (in Chinese) DOI: 10.3760/cma.j.cn112141-20200213-00101 |
| [6] |
|
| [7] |
Chinese Society of Hepatology, Chinese Medical Association. Consensus on the management of hepatitis B virus infection in women of childbearing age[J]. J Clin Hepatol, 2018, 34(6): 1176-1180. (in Chinese) DOI: 10.3969/j.issn.1001-5256.2018.06.008 |
| [8] |
Chinese Society of Hepatology, Chinese Medical Association, China Grade Center. 2019 Chinese practice guideline for prevention and treatment of hepatitis B virus mother-to-child transmission[J]. Chin J Infect Dis, 2019, 37(7): 388-396. https://www.cnki.com.cn/Article/CJFDTOTAL-LCGD202001019.htm |
| [9] |
ZHANG H, PAN CQ, PANG Q, et al. Telbivudine or lamivudine use in late pregnancy safely reduces perinatal transmission of hepatitis B virus in real-life practice[J]. Hepatology, 2014, 60(2): 468-476. DOI: 10.1002/hep.27034 |
| [10] |
CHEN HL, LEE CN, CHANG CH, et al. Efficacy of maternal tenofovir disoproxil fumarate in interrupting mother-to-infant transmission of hepatitis B virus[J]. Hepatology, 2015, 62(2): 375-386. DOI: 10.1002/hep.27837 |
| [11] |
PAN CQ, DUAN Z, DAI E, et al. Tenofovir to prevent hepatitis B transmission in mothers with high viral load[J]. N Engl J Med, 2016, 374(24): 2324-2334. DOI: 10.1056/NEJMoa1508660 |
| [12] |
JOURDAIN G, NGO-GIANG-HUONG N, HARRISON L, et al. Tenofovir versus placebo to prevent perinatal transmission of hepatitis B[J]. N Engl J Med, 2018, 378(10): 911-923. DOI: 10.1056/NEJMoa1708131 |
| [13] |
ZENG QL, YU ZJ, JI F, et al. Tenofovir alafenamide to prevent perinatal hepatitis B transmission: A multicenter, prospective, observational study[J]. Clin Infect Dis, 2021.[Online ahead of print]
|
| [14] |
DING Y, CAO L, ZHU L, et al. Efficacy and safety of tenofovir alafenamide fumarate for preventing mother-to-child transmission of hepatitis B virus: A national cohort study[J]. Aliment Pharmacol Ther, 2020, 52(8): 1377-1386. DOI: 10.1111/apt.16043 |
| [15] |
|
| [16] |
|
| [17] |
CUI F, WOODRING J, CHAN P, et al. Considerations of antiviral treatment to interrupt mother-to-child transmission of hepatitis B virus in China[J]. Int J Epidemiol, 2018, 47(5): 1529-1537. http://europepmc.org/abstract/MED/29757383 |
| [18] |
|
| [19] |
PAN CQ, ZOU HB, CHEN Y, et al. Cesarean section reduces perinatal transmission of hepatitis B virus infection from hepatitis B surface antigen-positive women to their infants[J]. Clin Gastroenterol Hepatol, 2013, 11(10): 1349-1355. http://www.ncbi.nlm.nih.gov/pubmed/23639606 |
| [20] |
|
| [21] |
|
| [22] |
SCHILLIE S, VELLOZZI C, REINGOLD A, et al. Prevention of hepatitis B virus infection in the United States: Recommendations of the advisory committee on immunization practices[J]. MMWR Recomm Rep, 2018, 67(1): 1-31. http://www.ncbi.nlm.nih.gov/pubmed/29939980 |
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
WEN WH, CHEN HL, SHIH TT, et al. Taiwan Study Group for the Prevention of Mother-to-Infant Transmission of HBV (PreMIT study). Long-term growth and bone development in children of HBV-infected mothers with and without fetal exposure to tenofovir disoproxil fumarate[J]. J Hepatol, 2020, 72(6): 1082-1087.
|



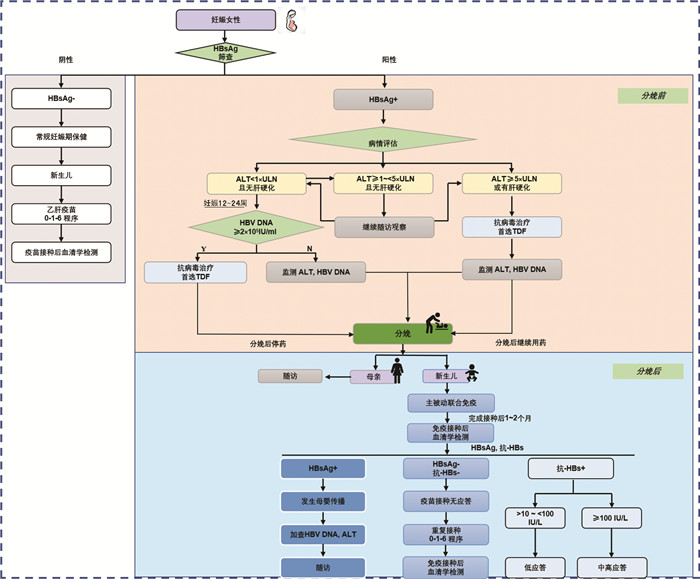




 DownLoad:
DownLoad: