| [1] |
SCHWEITZER A, HORN J, MIKOLAJCZYK RT, et al. Estimations of worldwide prevalence of chronic hepatitis B virus infection: A systematic review of data published between 1965 and 2013[J]. Lancet, 2015, 386(10003): 1546-1555. DOI: 10.1016/S0140-6736(15)61412-X. |
| [2] |
|
| [3] |
LIANG TJ, BLOCK TM, MCMAHON BJ, et al. Present and future therapies of hepatitis B: From discovery to cure[J]. Hepatology, 2015, 62(6): 1893-1908. DOI: 10.1002/hep.28025. |
| [4] |
SUNG JJ, WONG ML, BOWDEN S, et al. Intrahepatic hepatitis B virus covalently closed circular DNA can be a predictor of sustained response to therapy[J]. Gastroenterology, 2005, 128(7): 1890-1897. DOI: 10.1053/j.gastro.2005.03.009. |
| [5] |
WERLE-LAPOSTOLLE B, BOWDEN S, LOCARNINI S, et al. Persistence of cccDNA during the natural history of chronic hepatitis B and decline during adefovir dipivoxil therapy[J]. Gastroenterology, 2004, 126(7): 1750-1758. DOI: 10.1053/j.gastro.2004.03.018. |
| [6] |
GOVAN L, WU O, XIN Y, et al. Comparative effectiveness of antiviral treatment for hepatitis B: A systematic review and Bayesian network meta-analysis[J]. Eur J Gastroenterol Hepatol, 2015, 27(8): 882-894. DOI: 10.1097/MEG.0000000000000376. |
| [7] |
YE X, ZHOU M, HE Y, et al. Efficient inhibition of hepatitis B virus infection by a preS1-binding Peptide[J]. Sci Rep, 2016, 6: 29391. DOI: 10.1038/srep29391. |
| [8] |
KANEKO M, WATASHI K, KAMISUKI S, et al. A novel tricyclic polyketide, vanitaracin A, specifically inhibits the entry of hepatitis B and D viruses by targeting sodium taurocholate cotransporting polypeptide[J]. J Virol, 2015, 89(23): 11945-11953. DOI: 10.1128/JVI.01855-15. |
| [9] |
DONG Z, EKINS S, POLLI JE. Structure-activity relationship for FDA approved drugs as inhibitors of the human sodium taurocholate cotransporting polypeptide (NTCP)[J]. Mol Pharm, 2013, 10(3): 1008-1019. DOI: 10.1021/mp300453k. |
| [10] |
|
| [11] |
VOLZ T, ALLWEISS L, BEN MBAREK M, et al. The entry inhibitor Myrcludex-B efficiently blocks intrahepatic virus spreading in humanized mice previously infected with hepatitis B virus[J]. J Hepatol, 2013, 58(5): 861-867. DOI: 10.1016/j.jhep.2012.12.008. |
| [12] |
BLANK A, MARKERT C, HOHMANN N, et al. First-in-human application of the novel hepatitis B and hepatitis D virus entry inhibitor myrcludex B[J]. J Hepatol, 2016, 65(3): 483-489. DOI: 10.1016/j.jhep.2016.04.013. |
| [13] |
CARTHEW RW, SONTHEIMER EJ. Origins and Mechanisms of miRNAs and siRNAs[J]. Cell, 2009, 136(4): 642-655. DOI: 10.1016/j.cell.2009.01.035. |
| [14] |
YUEN MF, SCHIEFKE I, YOON JH, et al. RNA interference therapy with ARC-520 results in prolonged hepatitis B surface antigen response in patients with chronic hepatitis B infection[J]. Hepatology, 2020, 72(1): 19-31. DOI: 10.1002/hep.31008. |
| [15] |
GISH RG, YUEN MF, CHAN HL, et al. Synthetic RNAi triggers and their use in chronic hepatitis B therapies with curative intent[J]. Antiviral Res, 2015, 121: 97-108. DOI: 10.1016/j.antiviral.2015.06.019. |
| [16] |
|
| [17] |
LIU J, ZHANG S, WANG Q, et al. Seroepidemiology of hepatitis B virus infection in 2 million men aged 21-49 years in rural China: A population-based, cross-sectional study[J]. Lancet Infect Dis, 2016, 16(1): 80-86. DOI: 10.1016/S1473-3099(15)00218-2. |
| [18] |
FUNG J, WONG T, CHOK K, et al. Oral nucleos(t)ide analogs alone after liver transplantation in chronic hepatitis B with preexisting rt204 mutation[J]. Transplantation, 2017, 101(10): 2391-2398. DOI: 10.1097/TP.0000000000001883. |
| [19] |
ZHOU J, LIU YY, LIAN JS, et al. Efficacy and safety of tenofovir disoproxil treatment for chronic hepatitis B patients with genotypic resistance to other nucleoside analogues: A prospective study[J]. Chin Med J (Engl), 2017, 130(8): 914-919. DOI: 10.4103/0366-6999.204107. |
| [20] |
BERKE JM, DEHERTOGH P, VERGAUWEN K, et al. Capsid assembly modulators have a dual mechanism of action in primary human hepatocytes infected with hepatitis B virus[J]. Antimicrob Agents Chemother, 2017, 61(8). DOI: 10.1128/AAC.00560-17. |
| [21] |
KLUMPP K, LAM AM, LUKACS C, et al. High-resolution crystal structure of a hepatitis B virus replication inhibitor bound to the viral core protein[J]. Proc Natl Acad Sci U S A, 2015, 112(49): 15196-15201. DOI: 10.1073/pnas.1513803112. |
| [22] |
DEWITT MA, MAGIS W, BRAY NL, et al. Selection-free genome editing of the sickle mutation in human adult hematopoietic stem/progenitor cells[J]. Sci Transl Med, 2016, 8(360): 360ra134. DOI: 10.1126/scitranslmed.aaf9336. |
| [23] |
RAMANAN V, SHLOMAI A, COX DB, et al. CRISPR/Cas9 cleavage of viral DNA efficiently suppresses hepatitis B virus[J]. Sci Rep, 2015, 5: 10833. DOI: 10.1038/srep10833. |
| [24] |
WANG J, CHEN R, ZHANG R, et al. The gRNA-miRNA-gRNA ternary cassette combining CRISPR/Cas9 with RNAi approach strongly inhibits hepatitis B virus replication[J]. Theranostics, 2017, 7(12): 3090-3105. DOI: 10.7150/thno.18114. |
| [25] |
LOK AS, PAN CQ, HAN SH, et al. Randomized phase Ⅱ study of GS-4774 as a therapeutic vaccine in virally suppressed patients with chronic hepatitis B[J]. J Hepatol, 2016, 65(3): 509-516. DOI: 10.1016/j.jhep.2016.05.016. |
| [26] |
MENNE S, TUMAS DB, LIU KH, et al. Sustained efficacy and seroconversion with the Toll-like receptor 7 agonist GS-9620 in the Woodchuck model of chronic hepatitis B[J]. J Hepatol, 2015, 62(6): 1237-1245. DOI: 10.1016/j.jhep.2014.12.026. |
| [27] |
LANFORD RE, GUERRA B, CHAVEZ D, et al. GS-9620, an oral agonist of Toll-like receptor-7, induces prolonged suppression of hepatitis B virus in chronically infected chimpanzees[J]. Gastroenterology, 2013, 144(7): 1508-1517, 1517. e1-10. DOI: 10.1053/j.gastro.2013.02.003. |
| [28] |
GANE EJ, LIM YS, GORDON SC, et al. The oral toll-like receptor-7 agonist GS-9620 in patients with chronic hepatitis B virus infection[J]. J Hepatol, 2015, 63(2): 320-328. DOI: 10.1016/j.jhep.2015.02.037. |
| [29] |
JANSSEN H, BRUNETTO MR, KIM YJ, et al. Safety, efficacy and pharmacodynamics of vesatolimod (GS-9620) in virally suppressed patients with chronic hepatitis B[J]. J Hepatol, 2018, 68(3): 431-440. DOI: 10.1016/j.jhep.2017.10.027. |
| [30] |
SCHINAZI RF, EHTESHAMI M, BASSIT L, et al. Towards HBV curative therapies[J]. Liver Int, 2018, 38(Suppl 1): 102-114. DOI: 10.1111/liv.13656. |
| [31] |
SHIN YW, CHO DH, SONG GW, et al. A New ELISA to overcome the pitfalls in quantification of recombinant human monoclonal Anti-HBs, GC1102, by commercial immunoassays[J]. Biol Proced Online, 2018, 20: 18. DOI: 10.1186/s12575-018-0083-8. |
| [32] |
CHEN J, WANG XM, WU XJ, et al. Intrahepatic levels of PD-1/PD-L correlate with liver inflammation in chronic hepatitis B[J]. Inflamm Res, 2011, 60(1): 47-53. DOI: 10.1007/s00011-010-0233-1. |
| [33] |
GANE E, VERDON DJ, BROOKS AE, et al. Anti-PD-1 blockade with nivolumab with and without therapeutic vaccination for virally suppressed chronic hepatitis B: A pilot study[J]. J Hepatol, 2019, 71(5): 900-907. DOI: 10.1016/j.jhep.2019.06.028. |
| [34] |
BOCK CT, SCHRANZ P, SCHRZÖDER CH, et al. Hepatitis B virus genome is organized into nucleosomes in the nucleus of the infected cell[J]. Virus Genes, 1994, 8(3): 215-229. DOI: 10.1007/BF01703079. |
| [35] |
BOCK CT, SCHWINN S, LOCARNINI S, et al. Structural organization of the hepatitis B virus minichromosome[J]. J Mol Biol, 2001, 307(1): 183-196. DOI: 10.1006/jmbi.2000.4481. |
| [36] |
DAWSON MA. The cancer epigenome: Concepts, challenges, and therapeutic opportunities[J]. Science, 2017, 355(6330): 1147-1152. DOI: 10.1126/science.aam7304. |
| [37] |
GHOSH SK, PERRINE SP, WILLIAMS RM, et al. Histone deacetylase inhibitors are potent inducers of gene expression in latent EBV and sensitize lymphoma cells to nucleoside antiviral agents[J]. Blood, 2012, 119(4): 1008-1017. DOI: 10.1182/blood-2011-06-362434. |
| [38] |
BARLOW DP. Methylation and imprinting: From host defense to gene regulation?[J]. Science, 1993, 260(5106): 309-310. DOI: 10.1126/science.8469984. |
| [39] |
VIVEKANANDAN P, DANIEL HD, KANNANGAI R, et al. Hepatitis B virus replication induces methylation of both host and viral DNA[J]. J Virol, 2010, 84(9): 4321-4329. DOI: 10.1128/JVI.02280-09. |
| [40] |
SHIM YH, YOON GS, CHOI HJ, et al. p16 Hypermethylation in the early stage of hepatitis B virus-associated hepatocarcinogenesis[J]. Cancer Lett, 2003, 190(2): 213-219. DOI: 10.1016/s0304-3835(02)00613-4. |
| [41] |
ZHENG DL, ZHANG L, CHENG N, et al. Epigenetic modification induced by hepatitis B virus X protein via interaction with de novo DNA methyltransferase DNMT3A[J]. J Hepatol, 2009, 50(2): 377-387. DOI: 10.1016/j.jhep.2008.10.019. |
| [42] |
TROPBERGER P, MERCIER A, ROBINSON M, et al. Mapping of histone modifications in episomal HBV cccDNA uncovers an unusual chromatin organization amenable to epigenetic manipulation[J]. Proc Natl Acad Sci U S A, 2015, 112(42): e5715-e5724. DOI: 10.1073/pnas.1518090112. |
| [43] |
ZHANG W, CHEN J, WU M, et al. PRMT5 restricts hepatitis B virus replication through epigenetic repression of covalently closed circular DNA transcription and interference with pregenomic RNA encapsidation[J]. Hepatology, 2017, 66(2): 398-415. DOI: 10.1002/hep.29133. |




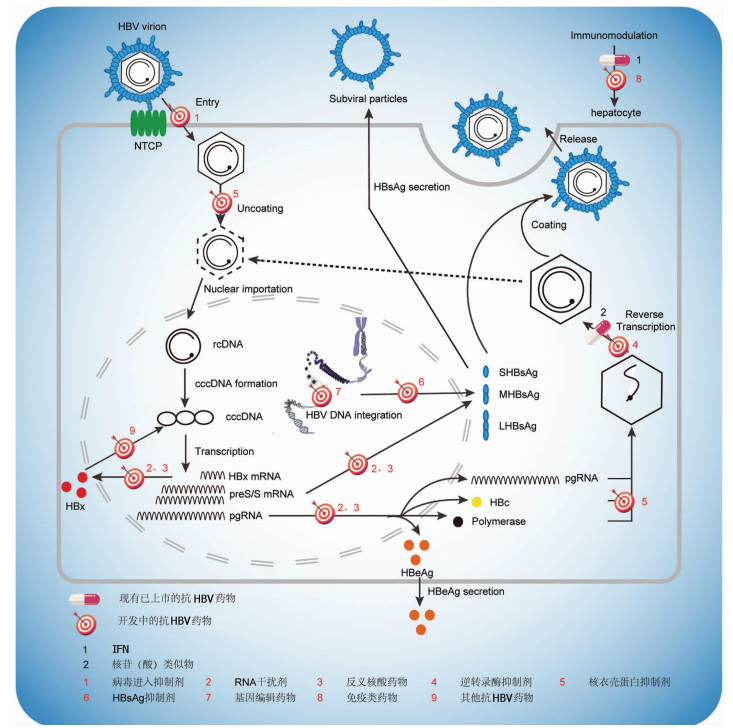




 DownLoad:
DownLoad: