| [1] |
SEEGER C, MASON WS. Molecular biology of hepatitis B virus infection[J]. Virology, 2015, 479-480: 672-686. DOI: 10.1016/j.virol.2015.02.031. |
| [2] |
WARD JW, HINMAN AR. What is needed to eliminate hepatitis B virus and hepatitis C virus as global health threats[J]. Gastroenterology, 2019, 156(2): 297-310. DOI: 10.1053/j.gastro.2018.10.048. |
| [3] |
|
| [4] |
BALKOW S, KERSTEN A, TRAN TT, et al. Concerted action of the FasL/Fas and perforin/granzyme A and B pathways is mandatory for the development of early viral hepatitis but not for recovery from viral infection[J]. J Virol, 2001, 75(18): 8781-8791. DOI: 10.1128/jvi.75.18.8781-8791.2001. |
| [5] |
FERRARI C. HBV and the immune response[J]. Liver Int, 2015, 35(Suppl 1): 121-128. DOI: 10.1111/liv.12749. |
| [6] |
PENNA A, DEL PRETE G, CAVALLI A, et al. Predominant T-helper 1 cytokine profile of hepatitis B virus nucleocapsid-specific T cells in acute self-limited hepatitis B[J]. Hepatology, 1997, 25(4): 1022-1027. DOI: 10.1002/hep.510250438. |
| [7] |
LANG J, NEUMANN-HAEFELIN C, THIMME R. Immunological cure of HBV infection[J]. Hepatol Int, 2019, 13(2): 113-124. DOI: 10.1007/s12072-018-9912-8. |
| [8] |
MENG Z, CHEN Y, LU M. Advances in targeting the innate and adaptive immune systems to cure chronic hepatitis B virus infection[J]. Front Immunol, 2019, 10: 3127. DOI: 10.3389/fimmu.2019.03127. |
| [9] |
ZHANG TY, YUAN Q, ZHAO JH, et al. Prolonged suppression of HBV in mice by a novel antibody that targets a unique epitope on hepatitis B surface antigen[J]. Gut, 2016, 65(4): 658-671. DOI: 10.1136/gutjnl-2014-308964. |
| [10] |
PAUL S, DICKSTEIN A, SAXENA A, et al. Role of surface antibody in hepatitis B reactivation in patients with resolved infection and hematologic malignancy: A meta-analysis[J]. Hepatology, 2017, 66(2): 379-388. DOI: 10.1002/hep.29082. |
| [11] |
EVENS AM, JOVANOVIC BD, SU YC, et al. Rituximab-associated hepatitis B virus (HBV) reactivation in lymphoproliferative diseases: Meta-analysis and examination of FDA safety reports[J]. Ann Oncol, 2011, 22(5): 1170-1180. DOI: 10.1093/annonc/mdq583. |
| [12] |
GINZBERG D, WONG RJ, GISH R. Global HBV burden: Guesstimates and facts[J]. Hepatol Int, 2018, 12(4): 315-329. DOI: 10.1007/s12072-018-9884-8. |
| [13] |
KEFALAKES H, BUDEUS B, WALKER A, et al. Adaptation of the hepatitis B virus core protein to CD8(+) T-cell selection pressure[J]. Hepatology, 2015, 62(1): 47-56. DOI: 10.1002/hep.27771. |
| [14] |
BLOCK TM, MEHTA AS, BLUMBERG BS, et al. Does rapid oligomerization of hepatitis B envelope proteins play a role in resistance to proteasome degradation and enhance chronicity?[J]. DNA Cell Biol, 2006, 25(3): 165-170. DOI: 10.1089/dna.2006.25.165. |
| [15] |
ATTANASIO J, WHERRY EJ. Costimulatory and coinhibitory receptor pathways in infectious disease[J]. Immunity, 2016, 44(5): 1052-1068. DOI: 10.1016/j.immuni.2016.04.022. |
| [16] |
|
| [17] |
WANG X, DONG Q, LI Q, et al. Dysregulated response of follicular helper T cells to hepatitis B surface antigen promotes HBV persistence in mice and associates with outcomes of patients[J]. Gastroenterology, 2018, 154(8): 2222-2236. DOI: 10.1053/j.gastro.2018.03.021. |
| [18] |
DU KY, YANG DL, LIU J. Regulatory T cells in hepatitis B[J]. J Prac Hepatol, 2019, 22(5): 613-616. DOI: 10.3969/j.issn.1672- 5069.2019.05.002.
杜科业, 杨东亮, 刘嘉. 慢性乙型肝炎与调节性T细胞研究进展[J]. 实用肝脏病杂志, 2019, 22(5): 613-616. DOI: 10.3969/j.issn.1672- 5069.2019.05.002.
|
| [19] |
LI K, LIU H, GUO T. Th17/Treg imbalance is an indicator of liver cirrhosis process and a risk factor for HCC occurrence in HBV patients[J]. Clin Res Hepatol Gastroenterol, 2017, 41(4): 399-407. DOI: 10.1016/j.clinre.2016.12.004. |
| [20] |
LIM CJ, LEE YH, PAN L, et al. Multidimensional analyses reveal distinct immune microenvironment in hepatitis B virus-related hepatocellular carcinoma[J]. Gut, 2019, 68(5): 916-927. DOI: 10.1136/gutjnl-2018-316510. |
| [21] |
OLIVIERO B, CERINO A, VARCHETTA S, et al. Enhanced B-cell differentiation and reduced proliferative capacity in chronic hepatitis C and chronic hepatitis B virus infections[J]. J Hepatol, 2011, 55(1): 53-60. DOI: 10.1016/j.jhep.2010.10.016. |
| [22] |
DAS A, ELLIS G, PALLANT C, et al. IL-10-producing regulatory B cells in the pathogenesis of chronic hepatitis B virus infection[J]. J Immunol, 2012, 189(8): 3925-3935. DOI: 10.4049/jimmunol.1103139. |
| [23] |
LIU Y, CHENG LS, WU SD, et al. IL-10-producing regulatory B-cells suppressed effector T-cells but enhanced regulatory T-cells in chronic HBV infection[J]. Clin Sci (Lond), 2016, 130(11): 907-919. DOI: 10.1042/CS20160069. |
| [24] |
van der MOLEN RG, SPRENGERS D, BINDA RS, et al. Functional impairment of myeloid and plasmacytoid dendritic cells of patients with chronic hepatitis B[J]. Hepatology, 2004, 40(3): 738-746. DOI: 10.1002/hep.20366. |
| [25] |
WOLTMAN AM, BOONSTRA A, JANSSEN HL. Dendritic cells in chronic viral hepatitis B and C: Victims or guardian angels?[J]. Gut, 2010, 59(1): 115-125. DOI: 10.1136/gut.2009.181040. |
| [26] |
FISICARO P, VALDATTA C, MASSARI M, et al. Antiviral intrahepatic T-cell responses can be restored by blocking programmed death-1 pathway in chronic hepatitis B[J]. Gastroenterology, 2010, 138(2): 682-693. DOI: 10.1053/j.gastro.2009.09.052. |
| [27] |
OUAGUIA L, LEROY V, DUFEU-DUCHESNE T, et al. Circulating and hepatic BDCA1 +, BDCA2 +, and BDCA3 + dendritic cells are differentially subverted in patients with chronic HBV infection[J]. Front Immunol, 2019, 10: 112. DOI: 10.3389/fimmu.2019.00112. |
| [28] |
LOK AS, PAN CQ, HAN SH, et al. Randomized phase Ⅱ study of GS-4774 as a therapeutic vaccine in virally suppressed patients with chronic hepatitis B[J]. J Hepatol, 2016, 65(3): 509-516. DOI: 10.1016/j.jhep.2016.05.016. |
| [29] |
BONI C, JANSSEN H, ROSSI M, et al. Combined GS-4774 and tenofovir therapy can improve HBV-specific T-cell responses in patients with chronic hepatitis[J]. Gastroenterology, 2019, 157(1): 227-241. e7. DOI: 10.1053/j.gastro.2019.03.044. |
| [30] |
YOON SK, SEO YB, IM SJ, et al. Safety and immunogenicity of therapeutic DNA vaccine with antiviral drug in chronic HBV patients and its immunogenicity in mice[J]. Liver Int, 2015, 35(3): 805-815. DOI: 10.1111/liv.12530. |
| [31] |
MARTIN P, DUBOIS C, JACQUIER E, et al. TG1050, an immunotherapeutic to treat chronic hepatitis B, induces robust T cells and exerts an antiviral effect in HBV-persistent mice[J]. Gut, 2015, 64(12): 1961-1971. DOI: 10.1136/gutjnl-2014-308041. |
| [32] |
ZOULIM F, FOURNIER C, HABERSETZER F, et al. Safety and immunogenicity of the therapeutic vaccine TG1050 in chronic hepatitis B patients: A phase 1b placebo-controlled trial[J]. Hum Vaccin Immunother, 2020, 16(2): 388-399. DOI: 10.1080/21645515.2019.1651141. |
| [33] |
AL MAHTAB M, AKBAR S, AGUILAR JC, et al. Treatment of chronic hepatitis B naïve patients with a therapeutic vaccine containing HBs and HBc antigens (a randomized, open and treatment controlled phase Ⅲ clinical trial)[J]. PLoS One, 2018, 13(8): e0201236. DOI: 10.1371/journal.pone.0201236. |
| [34] |
LAU GK, LOK AS, LIANG RH, et al. Clearance of hepatitis B surface antigen after bone marrow transplantation: Role of adoptive immunity transfer[J]. Hepatology, 1997, 25(6): 1497-1501. DOI: 10.1002/hep.510250631. |
| [35] |
BOHNE F, CHMIELEWSKI M, EBERT G, et al. T cells redirected against hepatitis B virus surface proteins eliminate infected hepatocytes[J]. Gastroenterology, 2008, 134(1): 239-247. DOI: 10.1053/j.gastro.2007.11.002. |
| [36] |
KREBS K, BÖTTINGER N, HUANG LR, et al. T cells expressing a chimeric antigen receptor that binds hepatitis B virus envelope proteins control virus replication in mice[J]. Gastroenterology, 2013, 145(2): 456-465. DOI: 10.1053/j.gastro.2013.04.047. |
| [37] |
KRUSE RL, SHUM T, TASHIRO H, et al. HBsAg-redirected T cells exhibit antiviral activity in HBV-infected human liver chimeric mice[J]. Cytotherapy, 2018, 20(5): 697-705. DOI: 10.1016/j.jcyt.2018.02.002. |
| [38] |
KAH J, KOH S, VOLZ T, et al. Lymphocytes transiently expressing virus-specific T cell receptors reduce hepatitis B virus infection[J]. J Clin Invest, 2017, 127(8): 3177-3188. DOI: 10.1172/JCI93024. |
| [39] |
LIU Q, TIAN Y, LI Y, et al. In vivo therapeutic effects of affinity-improved-TCR engineered T-cells on HBV-related hepatocellular carcinoma[J]. J Immunother Cancer, 2020, 8(2): e001748. DOI: 10.1136/jitc-2020-001748. |
| [40] |
QASIM W, BRUNETTO M, GEHRING AJ, et al. Immunotherapy of HCC metastases with autologous T cell receptor redirected T cells, targeting HBsAg in a liver transplant patient[J]. J Hepatol, 2015, 62(2): 486-491. DOI: 10.1016/j.jhep.2014.10.001. |
| [41] |
BERTOLETTI A. ImmTAV, a new immunotherapy targeting the source of HBV infection[J]. Hepatology, 2020, 72(5): 1514-1517. DOI: 10.1002/hep.31527. |
| [42] |
FERGUSSON JR, WALLACE Z, CONNOLLY MM, et al. Immune-mobilizing monoclonal T cell receptors mediate specific and rapid elimination of hepatitis B-infected cells[J]. Hepatology, 2020, 72(5): 1528-1540. DOI: 10.1002/hep.31503. |
| [43] |
AKBAR SM, CHEN S, AL-MAHTAB M, et al. Strong and multi-antigen specific immunity by hepatitis B core antigen (HBcAg)-based vaccines in a murine model of chronic hepatitis B: HBcAg is a candidate for a therapeutic vaccine against hepatitis B virus[J]. Antiviral Res, 2012, 96(1): 59-64. DOI: 10.1016/j.antiviral.2012.07.011. |
| [44] |
SUN HH, ZHOU DF, ZHOU JY. The role of DCs in the immunopathogenesis of chronic HBV infection and the methods of inducing DCs maturation[J]. J Med Virol, 2016, 88(1): 13-20. DOI: 10.1002/jmv.24306. |
| [45] |
MA YJ, HE M, HAN JA, et al. A clinical study of HBsAg-activated dendritic cells and cytokine-induced killer cells during the treatment for chronic hepatitis B[J]. Scand J Immunol, 2013, 78(4): 387-393. DOI: 10.1111/sji.12097. |
| [46] |
AKBAR SM, FURUKAWA S, HORⅡKE N, et al. Safety and immunogenicity of hepatitis B surface antigen-pulsed dendritic cells in patients with chronic hepatitis B[J]. J Viral Hepat, 2011, 18(6): 408-414. DOI: 10.1111/j.1365-2893.2010.01320.x. |
| [47] |
LIU J, ZHANG E, MA Z, et al. Enhancing virus-specific immunity in vivo by combining therapeutic vaccination and PD-L1 blockade in chronic hepadnaviral infection[J]. PLoS Pathog, 2014, 10(1): e1003856. DOI: 10.1371/journal.ppat.1003856. |
| [48] |
JACOBI FJ, WILD K, SMITS M, et al. OX40 stimulation and PD-L1 blockade synergistically augment HBV-specific CD4 T cells in patients with HBeAg-negative infection[J]. J Hepatol, 2019, 70(6): 1103-1113. DOI: 10.1016/j.jhep.2019.02.016. |
| [49] |
KAMPHORST AO, WIELAND A, NASTI T, et al. Rescue of exhausted CD8 T cells by PD-1-targeted therapies is CD28-dependent[J]. Science, 2017, 355(6332): 1423-1427. DOI: 10.1126/science.aaf0683. |
| [50] |
WEI SC, LEVINE JH, COGDILL AP, et al. Distinct cellular mechanisms underlie anti-CTLA-4 and anti-PD-1 checkpoint blockade[J]. Cell, 2017, 170(6): 1120-1133. e17. DOI: 10.1016/j.cell.2017.07.024. |
| [51] |
HUANG AC, POSTOW MA, ORLOWSKI RJ, et al. T-cell invigoration to tumour burden ratio associated with anti-PD-1 response[J]. Nature, 2017, 545(7652): 60-65. DOI: 10.1038/nature22079. |
| [52] |
GANE E, VERDON DJ, BROOKS AE, et al. Anti-PD-1 blockade with nivolumab with and without therapeutic vaccination for virally suppressed chronic hepatitis B: A pilot study[J]. J Hepatol, 2019, 71(5): 900-907. DOI: 10.1016/j.jhep.2019.06.028. |
| [53] |
BENGSCH B, JOHNSON AL, KURACHI M, et al. Bioenergetic insufficiencies due to metabolic alterations regulated by the inhibitory receptor PD-1 are an early driver of CD8(+) T cell exhaustion[J]. Immunity, 2016, 45(2): 358-373. DOI: 10.1016/j.immuni.2016.07.008. |
| [54] |
FISICARO P, BONI C, BARILI V, et al. Strategies to overcome HBV-specific T cell exhaustion: Checkpoint inhibitors and metabolic re-programming[J]. Curr Opin Virol, 2018, 30: 1-8. DOI: 10.1016/j.coviro.2018.01.003. |
| [55] |
FISICARO P, BARILI V, MONTANINI B, et al. Targeting mitochondrial dysfunction can restore antiviral activity of exhausted HBV-specific CD8 T cells in chronic hepatitis B[J]. Nat Med, 2017, 23(3): 327-336. DOI: 10.1038/nm.4275. |
| [56] |
ACERBI G, MONTALI I, FERRIGNO GD, et al. Functional reconstitution of HBV-specific CD8 T cells by in vitro polyphenol treatment in chronic hepatitis B[J]. J Hepatol, 2021, 74(4): 783-793. DOI: 10.1016/j.jhep.2020.10.034. |
| [57] |
GAO Y, ZHANG TY, YUAN Q, et al. Antibody-mediated immunotherapy against chronic hepatitis B virus infection[J]. Hum Vaccin Immunother, 2017, 13(8): 1768-1773. DOI: 10.1080/21645515.2017.1319021. |
| [58] |
|



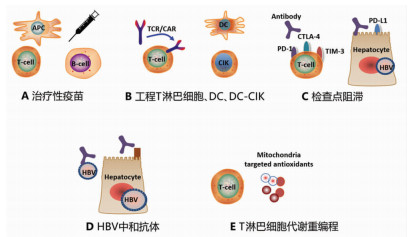




 DownLoad:
DownLoad: