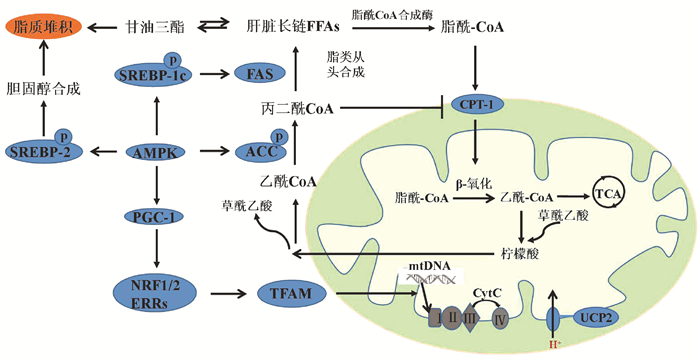
| [1] |
|
| [2] |
BOVERIS A, CHANCE B. The mitochondrial generation of hydrogen peroxide. General properties and effect of hyperbaric oxygen[J]. Biochem J, 1973, 134(3): 707-716. DOI: 10.1042/bj1340707. |
| [3] |
RIZKI G, ARNABOLDI L, GABRIELLI B, et al. Mice fed a lipogenic methionine-choline-deficient diet develop hypermetabolism coincident with hepatic suppression of SCD-1[J]. J Lipid Res, 2006, 47(10): 2280-2290. DOI: 10.1194/jlr.M600198-JLR200. |
| [4] |
SERVIDDIO G, GIUDETTI AM, BELLANTI F, et al. Oxidation of hepatic carnitine palmitoyl transferase-I (CPT-I) impairs fatty acid beta-oxidation in rats fed a methionine-choline deficient diet[J]. PLoS One, 2011, 6(9): e24084. DOI: 10.1371/journal.pone.0024084. |
| [5] |
ORELLANA-GAVALDÀ JM, HERRERO L, MALANDRINO MI, et al. Molecular therapy for obesity and diabetes based on a long-term increase in hepatic fatty-acid oxidation[J]. Hepatology, 2011, 53(3): 821-832. DOI: 10.1002/hep.24140 |
| [6] |
XIAO W, REN M, ZHANG C, et al. Amelioration of nonalcoholic fatty liver disease by hepatic stimulator substance via preservation of carnitine palmitoyl transferase-1 activity[J]. Am J Physiol Cell Physiol, 2015, 309(4): c215-c227. DOI: 10.1152/ajpcell.00133.2014. |
| [7] |
VERCAUTEREN K, GLEYZER N, SCARPULLA RC. Short hairpin RNA-mediated silencing of PRC (PGC-1-related coactivator) results in a severe respiratory chain deficiency associated with the proliferation of aberrant mitochondria[J]. J Biol Chem, 2009, 284(4): 2307-2319. DOI: 10.1074/jbc.M806434200. |
| [8] |
ZHANG Y, MA K, SONG S, et al. Peroxisomal proliferator-activated receptor-gamma coactivator-1 alpha (PGC-1 alpha) enhances the thyroid hormone induction of carnitine palmitoyltransferase I (CPT-I alpha)[J]. J Biol Chem, 2004, 279(52): 53963-53971. DOI: 10.1074/jbc.M406028200 |
| [9] |
SONG S, ATTIA RR, CONNAUGHTON S, et al. Peroxisome proliferator activated receptor alpha (PPARalpha) and PPAR gamma coactivator (PGC-1alpha) induce carnitine palmitoyltransferase IA (CPT-1A) via independent gene elements[J]. Mol Cell Endocrinol, 2010, 325(1-2): 54-63. DOI: 10.1016/j.mce.2010.05.019. |
| [10] |
BESSE-PATIN A, LÉVEILLÉ M, OROPEZA D, et al. Estrogen signals through peroxisome proliferator-activated receptor-γ coactivator 1α to reduce oxidative damage associated with diet-induced fatty liver disease[J]. Gastroenterology, 2017, 152(1): 243-256. DOI: 10.1053/j.gastro.2016.09.017. |
| [11] |
LEONE TC, LEHMAN JJ, FINCK BN, et al. PGC-1alpha deficiency causes multi-system energy metabolic derangements: muscle dysfunction, abnormal weight control and hepatic steatosis[J]. PLoS Biol, 2005, 3(4): e101. DOI: 10.1371/journal.pbio.0030101. |
| [12] |
MORRIS EM, MEERS GM, BOOTH FW, et al. PGC-1α overexpression results in increased hepatic fatty acid oxidation with reduced triacylglycerol accumulation and secretion[J]. Am J Physiol Gastrointest Liver Physiol, 2012, 303(8): G979-G992. DOI: 10.1152/ajpgi.00169.2012. |
| [13] |
BELLAFANTE E, MURZILLI S, SALVATORE L, et al. Hepatic-specific activation of peroxisome proliferator-activated receptor γ coactivator-1β protects against steatohepatitis[J]. Hepatology, 2013, 57(4): 1343-1356. DOI: 10.1002/hep.26222. |
| [14] |
DYCK JR, KUDO N, BARR AJ, et al. Phosphorylation control of cardiac acetyl-CoA carboxylase by cAMP-dependent protein kinase and 5'-AMP activated protein kinase[J]. Eur J Biochem, 1999, 262(1): 184-190. DOI: 10.1046/j.1432-1327.1999.00371.x. |
| [15] |
CHO YS, LEE JI, SHIN D, et al. Molecular mechanism for the regulation of human ACC2 through phosphorylation by AMPK[J]. Biochem Biophys Res Commun, 2010, 391(1): 187-192. DOI: 10.1016/j.bbrc.2009.11.029. |
| [16] |
ZHANG HA, YANG XY, XIAO YF. AMPKα1 overexpression alleviates the hepatocyte model of nonalcoholic fatty liver disease via inactivating p38MAPK pathway[J]. Biochem Biophys Res Commun, 2016, 474(2): 364-370. DOI: 10.1016/j.bbrc.2016.04.111. |
| [17] |
HORTON JD, GOLDSTEIN JL, BROWN MS. SREBPs: Activators of the complete program of cholesterol and fatty acid synthesis in the liver[J]. J Clin Invest, 2002, 109(9): 1125-1131. DOI: 10.1172/JCI15593. |
| [18] |
LI Y, XU S, MIHAYLOVA MM, et al. AMPK phosphorylates and inhibits SREBP activity to attenuate hepatic steatosis and atherosclerosis in diet-induced insulin-resistant mice[J]. Cell Metab, 2011, 13(4): 376-388. DOI: 10.1016/j.cmet.2011.03.009. |
| [19] |
WANG X, DONG LY, GAI QJ, et al. Lack of augmenter of liver regeneration disrupts cholesterol homeostasis of liver in mice by inhibiting the AMPK pathway[J]. Hepatol Commun, 2020, 4(8): 1149-1167. DOI: 10.1002/hep4.1532. |
| [20] |
WOODS A, WILLIAMS JR, MUCKETT PJ, et al. Liver-specific activation of AMPK prevents steatosis on a high-fructose diet[J]. Cell Rep, 2017, 18(13): 3043-3051. DOI: 10.1016/j.celrep.2017.03.011. |
| [21] |
JIANG Y, ZHANG H, DONG LY, et al. Increased hepatic UCP2 expression in rats with nonalcoholic steatohepatitis is associated with upregulation of Sp1 binding to its motif within the proximal promoter region[J]. J Cell Biochem, 2008, 105(1): 277-289. DOI: 10.1002/jcb.21827. |
| [22] |
CORTEZ-PINTO H, MACHADO MV. Uncoupling proteins and non-alcoholic fatty liver disease[J]. J Hepatol, 2009, 50(5): 857-860. DOI: 10.1016/j.jhep.2009.02.019. |
| [23] |
BAFFY G, ZHANG CY, GLICKMAN JN, et al. Obesity-related fatty liver is unchanged in mice deficient for mitochondrial uncoupling protein 2[J]. Hepatology, 2002, 35(4): 753-761. DOI: 10.1053/jhep.2002.32028. |
| [24] |
JEZEK P. Possible physiological roles of mitochondrial uncoupling proteins-UCPn[J]. Int J Biochem Cell Biol, 2002, 34(10): 1190-1206. DOI: 10.1016/s1357-2725(02)00061-4. |
| [25] |
CHAVIN KD, YANG S, LIN HZ, et al. Obesity induces expression of uncoupling protein-2 in hepatocytes and promotes liver ATP depletion[J]. J Biol Chem, 1999, 274(9): 5692-5700. DOI: 10.1074/jbc.274.9.5692. |
| [26] |
RIBEIRO PS, CORTEZ-PINTO H, SOLÁ S, et al. Hepatocyte apoptosis, expression of death receptors, and activation of NF-kappaB in the liver of nonalcoholic and alcoholic steatohepatitis patients[J]. Am J Gastroenterol, 2004, 99(9): 1708-1717. DOI: 10.1111/j.1572-0241.2004.40009.x. |
| [27] |
SERVIDDIO G, BELLANTI F, TAMBORRA R, et al. Uncoupling protein-2 (UCP2) induces mitochondrial proton leak and increases susceptibility of non-alcoholic steatohepatitis (NASH) liver to ischaemia-reperfusion injury[J]. Gut, 2008, 57(7): 957-965. DOI: 10.1136/gut.2007.147496. |
| [28] |
FÜLÖP P, DERDÁK Z, SHEETS A, et al. Lack of UCP2 reduces Fas-mediated liver injury in ob/ob mice and reveals importance of cell-specific UCP2 expression[J]. Hepatology, 2006, 44(3): 592-601. DOI: 10.1002/hep.21310. |
| [29] |
JIN X, YANG YD, CHEN K, et al. HDMCP uncouples yeast mitochondrial respiration and alleviates steatosis in L02 and hepG2 cells by decreasing ATP and H 2O 2 levels: A novel mechanism for NAFLD[J]. J Hepatol, 2009, 50(5): 1019-1028. DOI: 10.1016/j.jhep.2008.10.034. |
| [30] |
JIN X, LIU J, CHEN YP, et al. Effect of miR-146 targeted HDMCP up-regulation in the pathogenesis of nonalcoholic steatohepatitis[J]. PLoS One, 2017, 12(3): e0174218. DOI: 10.1371/journal.pone.0174218. |
| [31] |
MANSOURI A, GATTOLLIAT CH, ASSELAH T. Mitochondrial dysfunction and signaling in chronic liver diseases[J]. Gastroenterology, 2018, 155(3): 629-647. DOI: 10.1053/j.gastro.2018.06.083. |
| [32] |
SIMÕES I, FONTES A, PINTON P, et al. Mitochondria in non-alcoholic fatty liver disease[J]. Int J Biochem Cell Biol, 2018, 95: 93-99. DOI: 10.1016/j.biocel.2017.12.019. |
| [33] |
SVEGLIATI-BARONI G, PIERANTONELLI I, TORQUATO P, et al. Lipidomic biomarkers and mechanisms of lipotoxicity in non-alcoholic fatty liver disease[J]. Free Radic Biol Med, 2019, 144: 293-309. DOI: 10.1016/j.freeradbiomed.2019.05.029. |
| [34] |
KOLIAKI C, SZENDROEDI J, KAUL K, et al. Adaptation of hepatic mitochondrial function in humans with non-alcoholic fatty liver is lost in steatohepatitis[J]. Cell Metab, 2015, 21(5): 739-746. DOI: 10.1016/j.cmet.2015.04.004. |
| [35] |
GEORGE J, PERA N, PHUNG N, et al. Lipid peroxidation, stellate cell activation and hepatic fibrogenesis in a rat model of chronic steatohepatitis[J]. J Hepatol, 2003, 39(5): 756-764. DOI: 10.1016/s0168-8278(03)00376-3. |
| [36] |
XIAO WC, ZHANG J, CHEN SL, et al. Alleviation of palmitic acid-induced endoplasmic reticulum stress by augmenter of liver regeneration through IP3R-controlled Ca(2+) release[J]. J Cell Physiol, 2018, 233(8): 6148-6157. DOI: 10.1002/jcp.26463. |
| [37] |
XIAO F, ZHANG J, ZHANG C, et al. Hepatic stimulator substance inhibits calcium overflow through the mitochondria-associated membrane compartment during nonalcoholic steatohepatitis[J]. Lab Invest, 2017, 97(3): 289-301. DOI: 10.1038/labinvest.2016.139. |
| [38] |
SEKIYA M, HIRAISHI A, TOUYAMA M, et al. Oxidative stress induced lipid accumulation via SREBP1c activation in HepG2 cells[J]. Biochem Biophys Res Commun, 2008, 375(4): 602-607. DOI: 10.1016/j.bbrc.2008.08.068. |
| [39] |
WENZ T. Regulation of mitochondrial biogenesis and PGC-1α under cellular stress[J]. Mitochondrion, 2013, 13(2): 134-142. DOI: 10.1016/j.mito.2013.01.006. |
| [40] |
POPOV LD. Mitochondrial biogenesis: An update[J]. J Cell Mol Med, 2020, 24(9): 4892-4899. DOI: 10.1111/jcmm.15194. |
| [41] |
CAMERON RB, BEESON CC, SCHNELLMANN RG. Development of therapeutics that induce mitochondrial biogenesis for the treatment of acute and chronic degenerative diseases[J]. J Med Chem, 2016, 59(23): 10411-10434. DOI: 10.1021/acs.jmedchem.6b00669. |
| [42] |
TAHERZADEH-FARD E, SAFT C, AKKAD DA, et al. PGC-1alpha downstream transcription factors NRF-1 and TFAM are genetic modifiers of Huntington disease[J]. Mol Neurodegener, 2011, 6(1): 32. DOI: 10.1186/1750-1326-6-32. |
| [43] |
SCHREIBER SN, EMTER R, HOCK MB, et al. The estrogen-related receptor alpha (ERRalpha) functions in PPARgamma coactivator 1alpha (PGC-1alpha)-induced mitochondrial biogenesis[J]. Proc Natl Acad Sci U S A, 2004, 101(17): 6472-6477. DOI: 10.1073/pnas.0308686101. |
| [44] |
AHARONI-SIMON M, HANN-OBERCYGER M, PEN S, et al. Fatty liver is associated with impaired activity of PPARγ-coactivator 1α (PGC1α) and mitochondrial biogenesis in mice[J]. Lab Invest, 2011, 91(7): 1018-1028. DOI: 10.1038/labinvest.2011.55. |
| [45] |
FROMENTY B, GRIMBERT S, MANSOURI A, et al. Hepatic mitochondrial DNA deletion in alcoholics: Association with microvesicular steatosis[J]. Gastroenterology, 1995, 108(1): 193-200. DOI: 10.1016/0016-5085(95)90024-1. |
| [46] |
CIPOLAT S, MARTINS de BRITO O, DAL ZILIO B, et al. OPA1 requires mitofusin 1 to promote mitochondrial fusion[J]. Proc Natl Acad Sci U S A, 2004, 101(45): 15927-15932. DOI: 10.1073/pnas.0407043101. |
| [47] |
FILADI R, PENDIN D, PIZZO P. Mitofusin 2: From functions to disease[J]. Cell Death Dis, 2018, 9(3): 330. DOI: 10.1038/s41419-017-0023-6. |
| [48] |
ISHIHARA N, FUJITA Y, OKA T, et al. Regulation of mitochondrial morphology through proteolytic cleavage of OPA1[J]. EMBO J, 2006, 25(13): 2966-2977. DOI: 10.1038/sj.emboj.7601184. |
| [49] |
HALL AR, BURKE N, DONGWORTH RK, et al. Mitochondrial fusion and fission proteins: Novel therapeutic targets for combating cardiovascular disease[J]. Br J Pharmacol, 2014, 171(8): 1890-1906. DOI: 10.1111/bph.12516. |
| [50] |
HU C, HUANG Y, LI L. Drp1-dependent mitochondrial fission plays critical roles in physiological and pathological progresses in mammals[J]. Int J Mol Sci, 2017, 18(1): 144. DOI: 10.3390/ijms18010144. |
| [51] |
LOSÓN OC, SONG Z, CHEN H, et al. Fis1, Mff, MiD49, and MiD51 mediate Drp1 recruitment in mitochondrial fission[J]. Mol Biol Cell, 2013, 24(5): 659-667. DOI: 10.1091/mbc.E12-10-0721. |
| [52] |
GOMES LC, di BENEDETTO G, SCORRANO L. During autophagy mitochondria elongate, are spared from degradation and sustain cell viability[J]. Nat Cell Biol, 2011, 13(5): 589-598. DOI: 10.1038/ncb2220. |
| [53] |
HERNÁNDEZ-ALVAREZ MI, SEBASTIÁN D, VIVES S, et al. Deficient endoplasmic reticulum-mitochondrial phosphatidylserine transfer causes liver disease[J]. Cell, 2019, 177(4): 881-895. e17. DOI: 10.1016/j.cell.2019.04.010. |
| [54] |
LIU P, LIN H, XU Y, et al. Frataxin-mediated PINK1-parkin-dependent mitophagy in hepatic steatosis: The protective effects of quercetin[J]. Mol Nutr Food Res, 2018, 62(16): e1800164. DOI: 10.1002/mnfr.201800164. |
| [55] |
YAMADA T, MURATA D, ADACHI Y, et al. Mitochondrial stasis reveals p62-mediated ubiquitination in parkin-independent mitophagy and mitigates nonalcoholic fatty liver disease[J]. Cell Metab, 2018, 28(4): 588-604. e5. DOI: 10.1016/j.cmet.2018.06.014. |
| [56] |
ZHOU T, CHANG L, LUO Y, et al. Mst1 inhibition attenuates non-alcoholic fatty liver disease via reversing Parkin-related mitophagy[J]. Redox Biol, 2019, 21: 101120. DOI: 10.1016/j.redox.2019.101120. |
| [57] |
KUAN YC, HASHIDUME T, SHIBATA T, et al. Heat shock protein 90 modulates lipid homeostasis by regulating the stability and function of sterol regulatory element-binding protein (SREBP) and SREBP cleavage-activating protein[J]. J Biol Chem, 2017, 292(7): 3016-3028. DOI: 10.1074/jbc.M116.767277. |
| [58] |
WHEELER MC, GEKAKIS N. Hsp90 modulates PPARγ activity in a mouse model of nonalcoholic fatty liver disease[J]. J Lipid Res, 2014, 55(8): 1702-1710. DOI: 10.1194/jlr.M048918. |
| [59] |
BONORA M, PATERGNANI S, RAMACCINI D, et al. Physiopathology of the permeability transition pore: Molecular mechanisms in human pathology[J]. Biomolecules, 2020, 10(7): 998. doi: 10.3390/biom10070998. |
| [60] |
YIN X, ZHENG F, PAN Q, et al. Glucose fluctuation increased hepatocyte apoptosis under lipotoxicity and the involvement of mitochondrial permeability transition opening[J]. J Mol Endocrinol, 2015, 55(3): 169-181. DOI: 10.1530/JME-15-0101. |



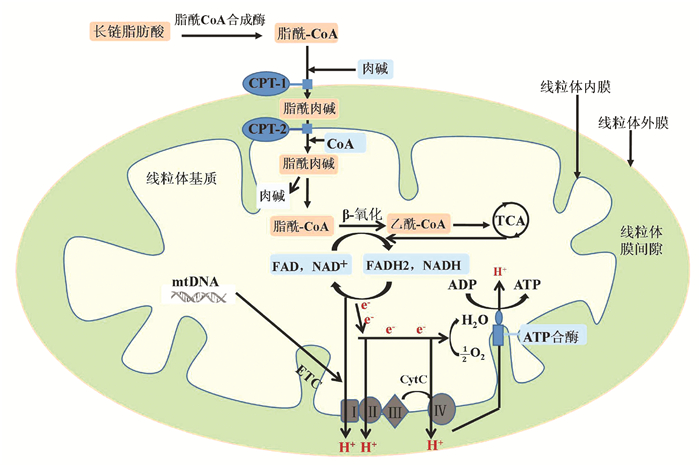




 DownLoad:
DownLoad: