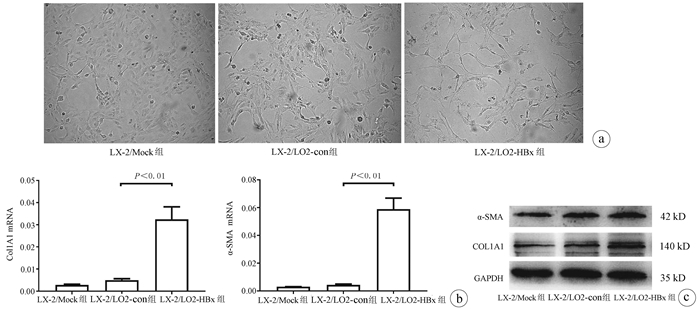
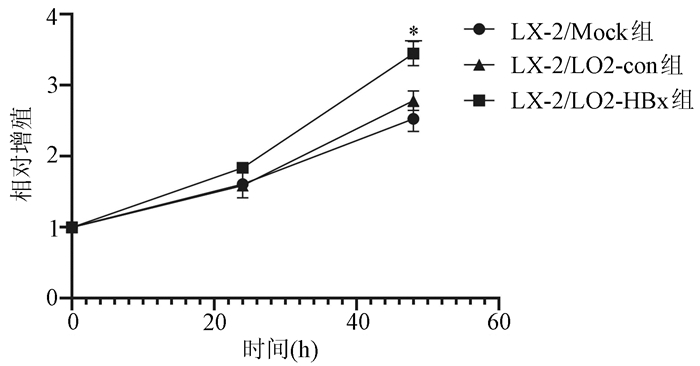
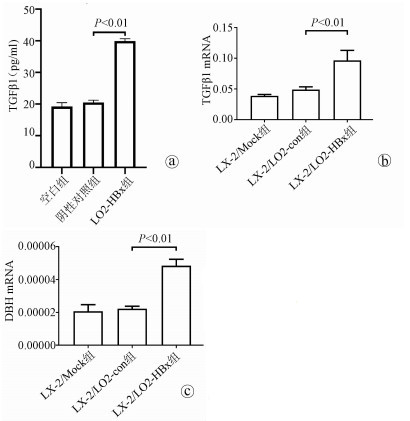
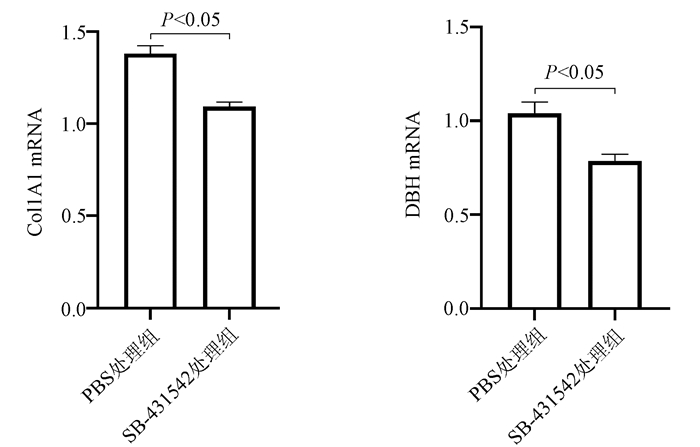
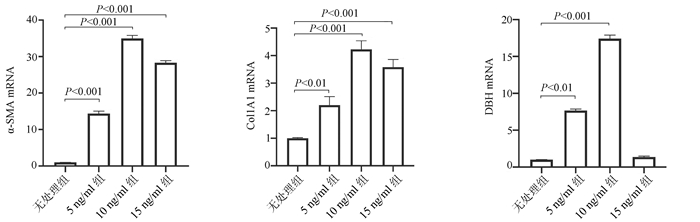
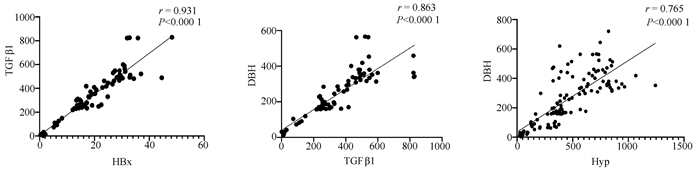
| [1] |
VITTAL A, GHANY MG. WHO guidelines for prevention, care and treatment of individuals infected with HBV: A US perspective[J]. Clin Liver Dis, 2019, 23(3): 417-432. DOI: 10.1016/j.cld.2019.04.008. |
| [2] |
WANG M, XI D, NING Q. Virus-induced hepatocellular carcinoma with special emphasis on HBV[J]. Hepatol Int, 2017, 11(2): 171-180. DOI: 10.1007/s12072-016-9779-5. |
| [3] |
BAGLIERI J, BRENNER DA, KISSELEVA T. The Role of fibrosis and liver-associated fibroblasts in the pathogenesis of hepatocellular carcinoma[J]. Int J Mol Sci, 2019, 20(7). DOI: 10.3390/ijms20071723. |
| [4] |
ZHU J, LUO Z, PAN Y, et al. H19/miR-148a/USP4 axis facilitates liver fibrosis by enhancing TGF-β signaling in both hepatic stellate cells and hepatocytes[J]. J Cell Physiol, 2019, 234(6): 9698-9710. DOI: 10.1002/jcp.27656. |
| [5] |
XU Y, ZHANG DQ, CHEN JM, et al. Effect of various cells on the activation of hepatic stellate cells in liver microenvironment[J]. J Clin Hepatol, 2019, 35(2): 424-430. DOI: 10.3969/j.issn.1001-5256.2019.02.042. |
| [6] |
EL-SERAG HB, MARRERO JA, RUDOLPH L, et al. Diagnosis and treatment of hepatocellular carcinoma[J]. Gastroenterology, 2008, 134(6): 1752-1763. DOI: 10.1053/j.gastro.2008.02.090. |
| [7] |
|
| [8] |
WEN X, HUAN H, WANG X, et al. Sympathetic neurotransmitters promote the process of recellularization in decellularized liver matrix via activating the IL-6/Stat3 pathway[J]. Biomed Mater, 2016, 11(6): 065007. DOI: 10.1088/1748-6041/11/6/065007. |
| [9] |
HUAN HB, WEN XD, CHEN XJ, et al. Sympathetic nervous system promotes hepatocarcinogenesis by modulating inflammation through activation of alpha1-adrenergic receptors of Kupffer cells[J]. Brain Behav Immun, 2017, 59: 118-134. DOI: 10.1016/j.bbi.2016.08.016. |
| [10] |
KAMIMURA K, INOUE R, NAGOYA T, et al. Autonomic nervous system network and liver regeneration[J]. World J Gastroenterol, 2018, 24(15): 1616-1621. DOI: 10.3748/wjg.v24.i15.1616. |
| [11] |
WANG L, ZHU L, WU K, et al. Mitochondrial general control of amino acid synthesis 5 like 1 regulates glutaminolysis, mammalian target of rapamycin complex 1 activity, and murine liver regeneration[J]. Hepatology, 2020, 71(2): 643-657. DOI: 10.1002/hep.30876. |
| [12] |
OBEN JA, ROSKAMS T, YANG S, et al. Hepatic fibrogenesis requires sympathetic neurotransmitters[J]. Gut, 2004, 53(3): 438-445. DOI: 10.1136/gut.2003.026658. |
| [13] |
COLL M, GENESCÀ J, RAURELL I, et al. Down-regulation of genes related to the adrenergic system may contribute to splanchnic vasodilation in rat portal hypertension[J]. J Hepatol, 2008, 49(1): 43-51. DOI: 10.1016/j.jhep.2008.03.015. |
| [14] |
LI P, WU G. Roles of dietary glycine, proline, and hydroxyproline in collagen synthesis and animal growth[J]. Amino Acids, 2018, 50(1): 29-38. DOI: 10.1007/s00726-017-2490-6. |
| [15] |
SRIVASTAVA AK, KHARE P, NAGAR HK, et al. Hydroxyproline: A potential biochemical marker and its role in the pathogenesis of different diseases[J]. Curr Protein Pept Sci, 2016, 17(6): 596-602. DOI: 10.2174/1389203717666151201192247. |
| [16] |
SHIN SK, KIM KO, KIM SH, et al. Exogenous 8-hydroxydeoxyguanosine ameliorates liver fibrosis through the inhibition of Rac1-NADPH oxidase signaling[J]. J Gastroenterol Hepatol, 2020, 35(6): 1078-1087. DOI: 10.1111/jgh.14979. |



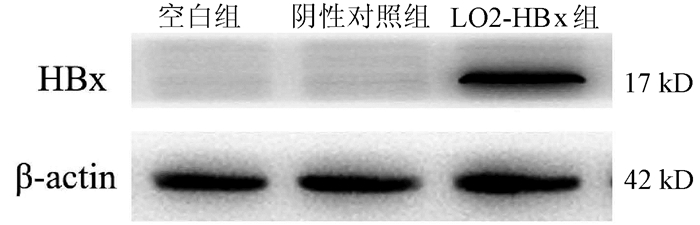




 DownLoad:
DownLoad: