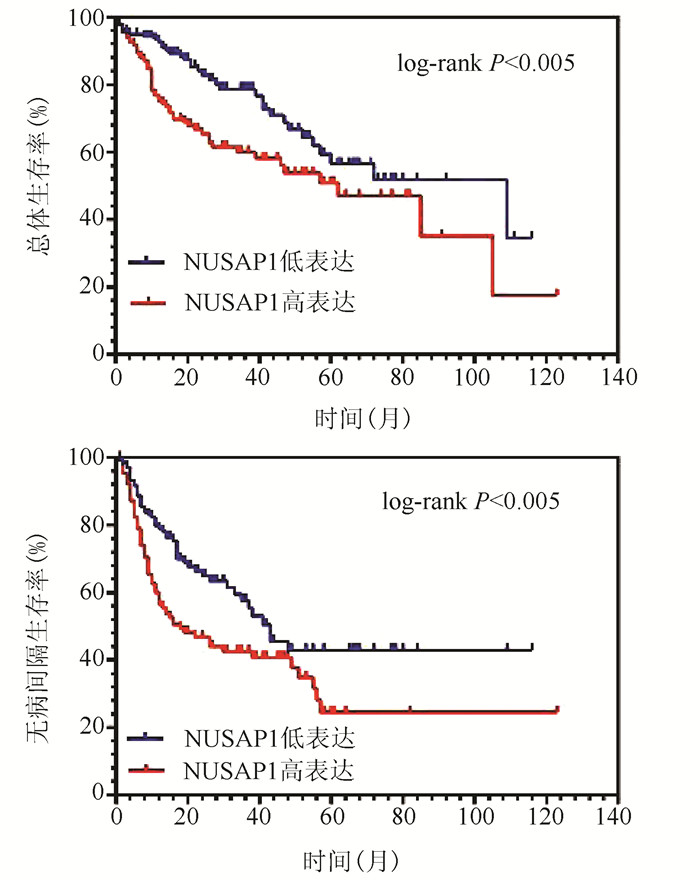
| [1] |
BRAY F, FERLAY J, SOERJOMATARAM I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA Cancer J Clin, 2018, 68(6): 394-424. DOI: 10.3322/caac.21492. |
| [2] |
KULIK L, EL-SERAG HB. Epidemiology and management of hepatocellular carcinoma[J]. Gastroenterology, 2019, 156(2): 477-491. e1. DOI: 10.1053/j.gastro.2018.08.065. |
| [3] |
WANG SY, FENG LY, MENG ZQ. Bicluster and pathway enrichment analysis related to tumor progression of hepatocellular carcinoma[J]. Eur Rev Med Pharmacol Sci, 2015, 19(7): 1191-1197. http://europepmc.org/articles/PMC4237098 |
| [4] |
BAATARKHUU O, GERELCHIMEG T, MUNKH-ORSHIKH D, et al. Epidemiology, genotype distribution, prognosis, control, and management of viral hepatitis B, C, D, and hepatocellular carcinoma in mongolia[J]. Euroasian J Hepatogastroenterol, 2018, 8(1): 57-62. DOI: 10.5005/jp-journals-10018-1260. |
| [5] |
EL-SERAG HB. Epidemiology of viral hepatitis and hepatocellular carcinoma[J]. Gastroenterology, 2012, 142(6): 1264-1273. e1. DOI: 10.1053/j.gastro.2011.12.061. |
| [6] |
|
| [7] |
RAEMAEKERS T, RIBBECK K, BEAUDOUIN J, et al. NuSAP, a novel microtubule-associated protein involved in mitotic spindle organization[J]. J Cell Biol, 2003, 162(6): 1017-1029. DOI: 10.1083/jcb.200302129. |
| [8] |
|
| [9] |
ZHOU Q, LEE KJ, KURASAWA Y, et al. Faithful chromosome segregation in Trypanosoma brucei requires a cohort of divergent spindle-associated proteins with distinct functions[J]. Nucleic Acids Res, 2018, 46(16): 8216-8231. DOI: 10.1093/nar/gky557. |
| [10] |
LIU Z, GUAN C, LU C, et al. High NUSAP1 expression predicts poor prognosis in colon cancer[J]. Pathol Res Pract, 2018, 214(7): 968-973. DOI: 10.1016/j.prp.2018.05.017. |
| [11] |
|
| [12] |
GAO S, YIN H, TONG H, et al. Nucleolar and spindle associated protein 1 (NUSAP1) promotes bladder cancer progression through the TGF-β signaling pathway[J]. Onco Targets Ther, 2020, 13: 813-825. DOI: 10.2147/OTT.S237127. |
| [13] |
GE Y, LI Q, LIN L, et al. Downregulation of NUSAP1 suppresses cell proliferation, migration, and invasion via inhibiting mTORC1 signalling pathway in gastric cancer[J]. Cell Biochem Funct, 2020, 38(1): 28-37. DOI: 10.1002/cbf.3444. |
| [14] |
GORDON CA, GULZAR ZG, BROOKS JD. NUSAP1 expression is upregulated by loss of RB1 in prostate cancer cells[J]. Prostate, 2015, 75(5): 517-526. DOI: 10.1002/pros.22938. |
| [15] |
ZHANG X, PAN Y, FU H, et al. Nucleolar and spindle associated protein 1 (NUSAP1) inhibits cell proliferation and enhances susceptibility to epirubicin in invasive breast cancer cells by regulating cyclin D kinase (CDK1) and DLGAP5 expression[J]. Med Sci Monit, 2018, 24: 8553-8564. DOI: 10.12659/MSM.910364. |
| [16] |
XIE Q, OU-YANG W, ZHANG M, et al. Decreased expression of NUSAP1 predicts poor overall survival in cervical cancer[J]. J Cancer, 2020, 11(10): 2852-2863. DOI: 10.7150/jca.34640. |
| [17] |
LI Y, HUANG C, DING L, et al. Deep learning in bioinformatics: Introduction, application, and perspective in the big data era[J]. Methods, 2019, 166: 4-21. DOI: 10.1016/j.ymeth.2019.04.008. |
| [18] |
SONG B, TANG JW, WANG B, et al. Identify lymphatic metastasis-associated genes in mouse hepatocarcinoma cell lines using gene chip[J]. World J Gastroenterol, 2005, 11(10): 1463-1472. DOI: 10.3748/wjg.v11.i10.1463. |
| [19] |
ZHOU YL, LIU JY, YAO HW, et al. Increased expression of lncFOXC1 promotes liver cancer formation[J]. Chin J Clin Pharmacol Ther, 2019, 24(1): 27-31. DOI: 10.12092/j.issn.1009-2501.2019.01.005. |
| [20] |
|










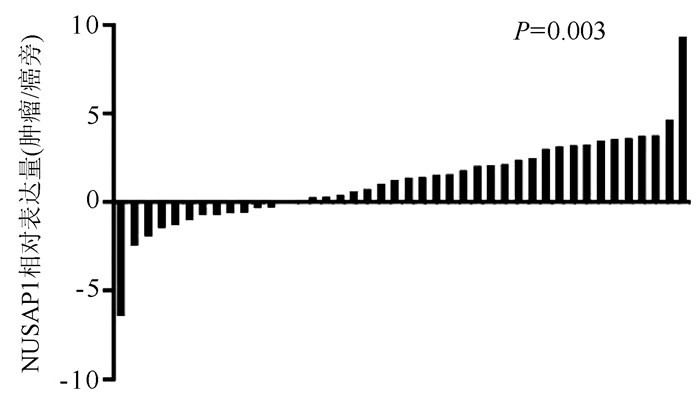




 DownLoad:
DownLoad: