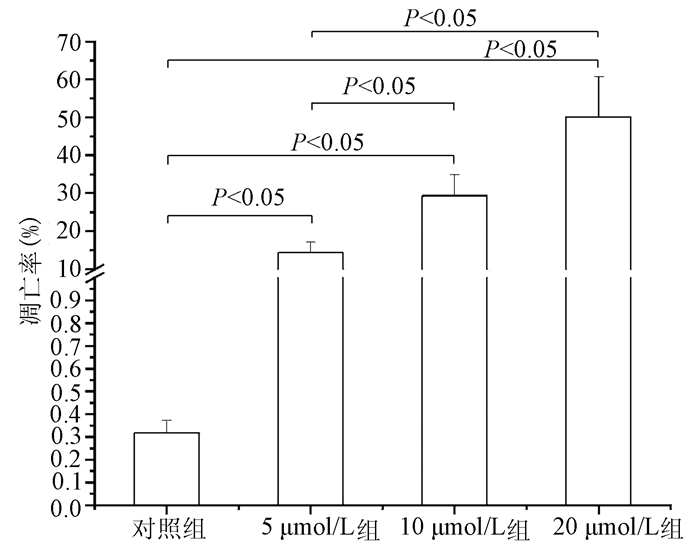
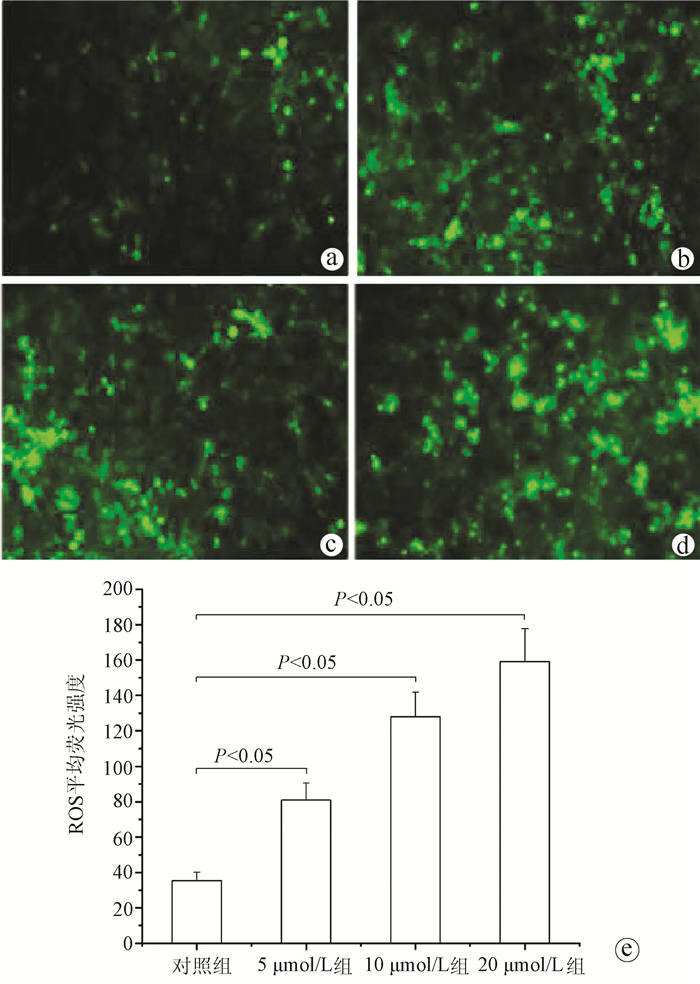
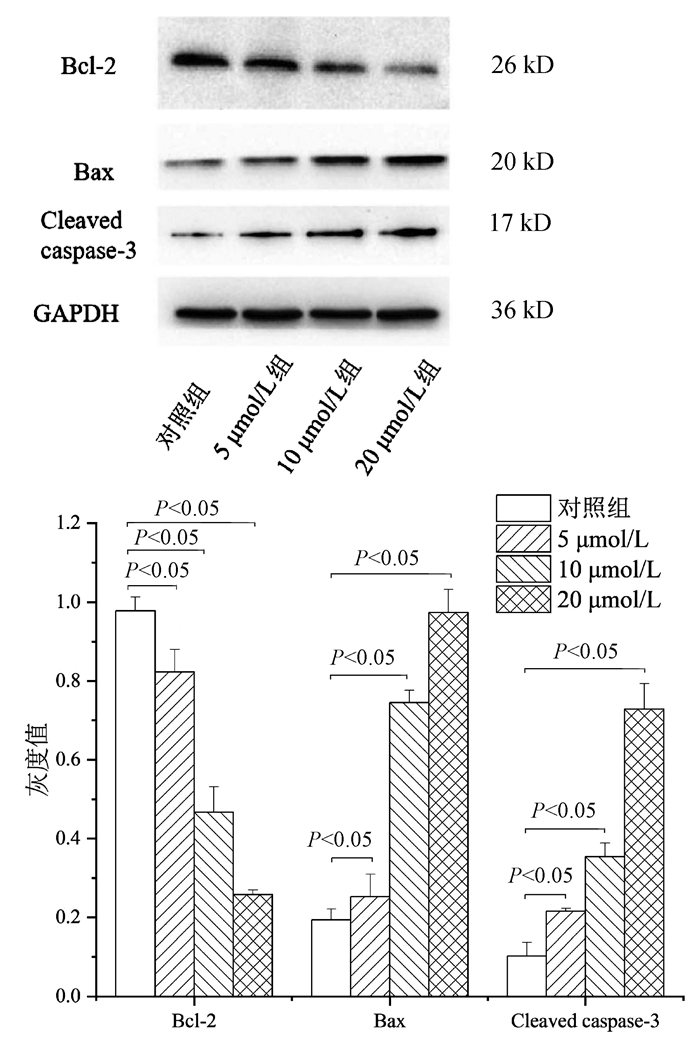
| [1] |
SUNG H, FERLAY J, SIEGEL RL, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA Cancer J Clin, 2021, 71(3): 209-249. DOI: 10.3322/caac.21660. |
| [2] |
WU YX, SHENG QS. Advances in traditional Chinese medicine therapy for primary hepatocarcinoma[J]. Guangxi Med J, 2020, 42(4): 483-485. DOI: 10.1165/j.issn.0253-4304.2020.04.26.
|
| [3] |
LI ZW. Analysis of postoperative prognosis and risk factors in patients with primary liver cancer[J]. China's Naturopathy, 2020, 28(13): 73-75. DOI: 10.19621/j.cnki.11-3555/r.2020.1334. |
| [4] |
FORNER A, REIG ME, de LOPE CR, et al. Current strategy for staging and treatment: The BCLC update and future prospects[J]. Semin Liver Dis, 2010, 30(1): 61-74. DOI: 10.1055/s-0030-1247133. |
| [5] |
HUANG JH, HUANG ZM, ZHANG TQ, et al. Progress in comprehensive treatment of advanced liver cancer based on interventional therapy[J/CD]. Electronic J Liver Tumor, 2019, 6(4): 27-31. DOI: 10.3969/j.issn.2095-7815.2019.04.007. |
| [6] |
|
| [7] |
ZHAN YP, ZHAI XF. Study on prescription compatibility law of contemporary famous doctors of TCM in treating primary liver cancer based on data mining[J]. J Liaoning Univ Tradit Chin Med, 2018, 20(3): 159-162. DOI: 10.13194/j.issn.1673-842x.2018.03.046. |
| [8] |
ZHANG Y, CHEN HG, ZHAO C, et al. Research progress on anti-hepatocellular carcinoma mechanism of active ingredients of traditional Chinese medicine[J]. China J Chin Mater Med, 2020, 45(14): 3395-3406. DOI: 10.19540/j.cnki.cjcmm.20200429.601. |
| [9] |
|
| [10] |
|
| [11] |
CHEN TY, LIU YL, HOU TL, et al. Study on the protective effect of atractylone on acute lung injury in mice[J]. Mod J Integr Tradit Chin West Med, 2018, 27(24): 2623-2626. DOI: 10.3969/j.issn.1008-8849.2018.24.001. |
| [12] |
ATHINARAYANAN S, FAN YY, WANG X, et al. Fatty acid desaturase 1 influences hepatic lipid homeostasis by modulating the PPARα-FGF21 axis[J]. Hepatol Commun, 2021, 5(3): 461-477. DOI: 10.1002/hep4.1629. |
| [13] |
SUN YP, CHENG Z, WU Z, et al. Research on the inheritance, innovation and high-quality development of Chinese herbal medicine Atractylodes[J]. Hubei Agricult Sci, 2020, 59(11): 203-207. DOI: 10.14088/j.cnki.issn0439-8114.2020.11.040. |
| [14] |
ZHANG MF, SHEN YQ. Advances in studies on anti-inflammation, antitumor, and immunoregulation of Atractylodis Rhizoma[J]. Drug Eval Res, 2016, 39(5): 885-890. DOI: 10.7501/j.issn.1674-6376.2016.05.037. |
| [15] |
ZHOU Y, LU JT, ZHANG XD, et al. Potential target prediction and forward molecular docking verification of atractylon[J]. J Shaoyang Uni(Natural Science Edition), 2019, 16(1): 98-104. DOI: CNKI:SUN:SYXZ.0.2019-01-013. |
| [16] |
GENG W, LIANG W, YE ZB, et al. Mechanism of HT29 apoptosis in colorectal cancer cells by atractylone[J]. Chin Tradit Patent Med, 2018, 40(4): 937-940. DOI: 10.3969/j.issn.1001-1528.2018.04.034. |
| [17] |
|
| [18] |
SHAHAR N, LARISCH S. Inhibiting the inhibitors: Targeting anti-apoptotic proteins in cancer and therapy resistance[J]. Drug Resist Updat, 2020, 52: 100712. DOI: 10.1016/j.drup.2020.100712. |
| [19] |
QIAO Z, CHENG Y, LIU S, et al. Casticin inhibits esophageal cancer cell proliferation and promotes apoptosis by regulating mitochondrial apoptotic and JNK signaling pathways[J]. Naunyn Schmiedebergs Arch Pharmacol, 2019, 392(2): 177-187. DOI: 10.1007/s00210-018-1574-5. |
| [20] |
ZHANG Y, WANG Y, ZHAO Y, et al. Novel camphor-based pyrimidine derivatives induced cancer cell death through a ROS-mediated mitochondrial apoptosis pathway[J]. RSC Adv, 2019, 9(51): 29711-29720. DOI: 10.1039/C9RA05900H. |
| [21] |
ZHONG FR, CHENG HL, ZHANG H, et al. Effect of kaempferol on the proliferation, migration, invasion, and apoptosis of human hepatoma Bel-7402 cells[J]. J Clin Hepatol, 2020, 36(12): 2725-2729. DOI: 10.3969/j.issn.1001-5256.2020.12.017. |
| [22] |
LI R, ZOU X, ZHU T, et al. Destruction of neutrophil extracellular traps promotesthe apoptosis and inhibits the invasion of gastric cancer cells by regulating the expression of Bcl-2, Bax and NF-κB[J]. Onco Targets Ther, 2020, 13: 5271-5281. DOI: 10.2147/OTT.S227331. |
| [23] |
|
| [24] |
GUO DF, WANG RT, HUANG JC, et al. Curcumin derivative C086 induces the apoptosis of hepatoma HepG2 cells via the PI3K-Akt pathway[J]. Chin J Gerontol, 2021, 41(6): 1270-1274. DOI: 10.3969/j.issn.1005-9202.2021.06.042. |
| [25] |
WU XY, ZHAO YN, WANG XJ, et al. Effect of galectin-3 expression suppression on expressions of Bcl-2 and Bax in gastric cancer MGC-803 cells and its promotion on apoptosis[J]. J Jilin Univ(Med Edit), 2020, 46(2): 335-339. DOI: 10.13481/j.1671-587x.20200221. |
| [26] |
FU K, SUI GC, SHI JM. Inhibitory effect of triterpenoid saponins from Dioscorea gracillima on human hepatoma HepG2 cells[J/CD]. Cardiovasc Dis J Integr Tradit Chin Western Med (Electronic), 2020, 8(1): 67-68. DOI: 10.16282/j.cnki.cn11-9336/r.2020.01.052. |
| [27] |
NIE SS, LI X, ZHAO YH, et al. Effect of modified Si Junzitang drug serum on expression of apoptosis-related molecules of gastric cancer cell SGC-7901[J]. Chin J Exp Med Formul, 2019, 25(9): 25-30. DOI: 10.13422/j.cnki.syfjx.20190824. |
| [28] |
MAO M, HUA Y, JIANG X, et al. Expression of tumor necrosis factor alpha and neuronal apoptosis in the developing rat brain after neonatal stroke[J]. Neurosci Lett, 2006, 403(3): 227-232. DOI: 10.1016/j.neulet.2006.03.078. |



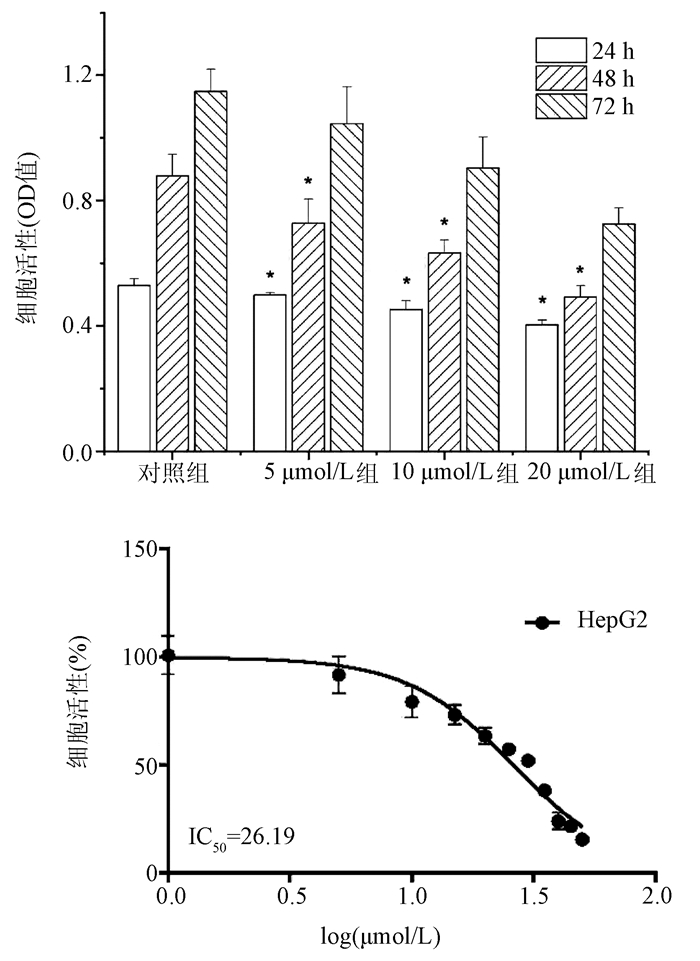




 DownLoad:
DownLoad: