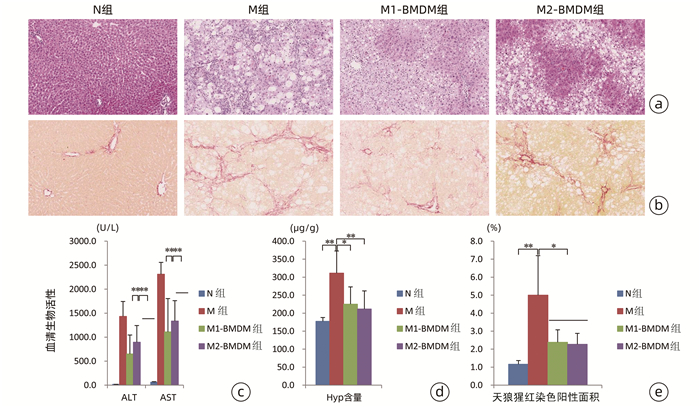
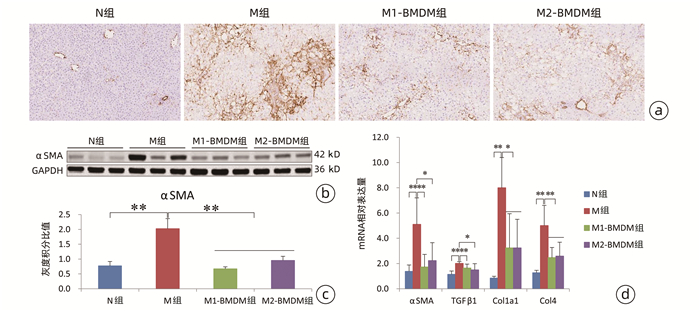
| [1] |
RAMACHANDRAN P, DOBIE R, WILSON-KANAMORI JR, et al. Resolving the fibrotic niche of human liver cirrhosis at single-cell level[J]. Nature, 2019, 575(7783): 512-518. DOI: 10.1038/s41586-019-1631-3. |
| [2] |
KARLMARK KR, WEISKIRCHEN R, ZIMMERMANN HW, et al. Hepatic recruitment of the inflammatory Gr1+ monocyte subset upon liver injury promotes hepatic fibrosis[J]. Hepatology, 2009, 50(1): 261-274. DOI: 10.1002/hep.22950. |
| [3] |
|
| [4] |
ORECCHIONI M, GHOSHEH Y, PRAMOD AB, et al. Macrophage polarization: Different gene signatures in M1(LPS+) vs. classically and M2(LPS-) vs. alternatively activated macrophages[J]. Front Immunol, 2019, 10: 1084. DOI: 10.3389/fimmu.2019.01084. |
| [5] |
MURRAY PJ, ALLEN JE, BISWAS SK, et al. Macrophage activation and polarization: Nomenclature and experimental guidelines[J]. Immunity, 2014, 41(1): 14-20. DOI: 10.1016/j.immuni.2014.06.008. |
| [6] |
|
| [7] |
WATANABE Y, TSUCHIYA A, SEINO S, et al. Mesenchymal stem cells and induced bone marrow-derived macrophages synergistically improve liver fibrosis in mice[J]. Stem Cells Transl Med, 2019, 8(3): 271-284. DOI: 10.1002/sctm.18-0105. |
| [8] |
PINEDA-TORRA I, GAGE M, de JUAN A, et al. Isolation, culture, and polarization of murine bone marrow-derived and peritoneal macrophages[J]. Methods Mol Biol, 2015, 1339: 101-109. DOI: 10.1007/978-1-4939-2929-0_6. |
| [9] |
MILY A, KALSUM S, LORETI MG, et al. Polarization of M1 and M2 human monocyte-derived cells and analysis with flow cytometry upon mycobacterium tuberculosis infection[J]. J Vis Exp, 2020, (163). DOI: 10.3791/61807. |
| [10] |
JAMALL IS, FINELLI VN, QUE HEE SS. A simple method to determine nanogram levels of 4-hydroxyproline in biological tissues[J]. Anal Biochem, 1981, 112(1): 70-75. DOI: 10.1016/0003-2697(81)90261-x. |
| [11] |
MU YP, OGAWA T, KAWADA N. Reversibility of fibrosis, inflammation, and endoplasmic reticulum stress in the liver of rats fed a methionine-choline-deficient diet[J]. Lab Invest, 2010, 90(2): 245-256. DOI: 10.1038/labinvest.2009.123. |
| [12] |
PRADERE JP, KLUWE J, DE MINICIS S, et al. Hepatic macrophages but not dendritic cells contribute to liver fibrosis by promoting the survival of activated hepatic stellate cells in mice[J]. Hepatology, 2013, 58(4): 1461-1473. DOI: 10.1002/hep.26429. |
| [13] |
HUME DA, IRVINE KM, PRIDANS C. The mononuclear phagocyte system: The relationship between monocytes and macrophages[J]. Trends Immunol, 2019, 40(2): 98-112. DOI: 10.1016/j.it.2018.11.007. |
| [14] |
MA PF, GAO CC, YI J, et al. Cytotherapy with M1-polarized macrophages ameliorates liver fibrosis by modulating immune microenvironment in mice[J]. J Hepatol, 2017, 67(4): 770-779. DOI: 10.1016/j.jhep.2017.05.022. |
| [15] |
FRIEDMAN SL. Hepatic stellate cells: Protean, multifunctional, and enigmatic cells of the liver[J]. Physiol Rev, 2008, 88(1): 125-172. DOI: 10.1152/physrev.00013.2007. |
| [16] |
NOVO E, MARRA F, ZAMARA E, et al. Dose dependent and divergent effects of superoxide anion on cell death, proliferation, and migration of activated human hepatic stellate cells[J]. Gut, 2006, 55(1): 90-97. DOI: 10.1136/gut.2005.069633. |
| [17] |
RAMACHANDRAN P, IREDALE JP. Macrophages: Central regulators of hepatic fibrogenesis and fibrosis resolution[J]. J Hepatol, 2012, 56(6): 1417-1419. DOI: 10.1016/j.jhep.2011.10.026. |
| [18] |
FENG M, DING J, WANG M, et al. Kupffer-derived matrix metalloproteinase-9 contributes to liver fibrosis resolution[J]. Int J Biol Sci, 2018, 14(9): 1033-1040. DOI: 10.7150/ijbs.25589. |
| [19] |
GUO J, LUO Y, YIN F, et al. Overexpression of tumor necrosis factor-like ligand 1 A in myeloid cells aggravates liver fibrosis in mice[J]. J Immunol Res, 2019, 2019: 7657294. DOI: 10.1155/2019/7657294. |
| [20] |
GORDON S, MARTINEZ FO. Alternative activation of macrophages: Mechanism and functions[J]. Immunity, 2010, 32(5): 593-604. DOI: 10.1016/j.immuni.2010.05.007. |
| [21] |
TAO Y, WANG M, CHEN E, et al. Liver Regeneration: Analysis of the main relevant signaling molecules[J]. Mediators Inflamm, 2017, 2017: 4256352. DOI: 10.1155/2017/4256352. |
| [22] |
CRESSMAN DE, GREENBAUM LE, DEANGELIS RA, et al. Liver failure and defective hepatocyte regeneration in interleukin-6-deficient mice[J]. Science, 1996, 274(5291): 1379-1383. DOI: 10.1126/science.274.5291.1379. |
| [23] |
TAUB R. Liver regeneration: From myth to mechanism[J]. Nat Rev Mol Cell Biol, 2004, 5(10): 836-847. DOI: 10.1038/nrm1489. |
| [24] |
KANG LI, MARS WM, MICHALOPOULOS GK. Signals and cells involved in regulating liver regeneration[J]. Cells, 2012, 1(4): 1261-1292. DOI: 10.3390/cells1041261. |
| [25] |
ELCHANINOV AV, FATKHUDINOV TK, VISHNYAKOVA PA, et al. Phenotypical and functional polymorphism of liver resident macrophages[J]. Cells, 2019, 8(9): 1032-1053. DOI: 10.3390/cells8091032. |
| [26] |
CAMPANA L, ESSER H, HUCH M, et al. Liver regeneration and inflammation: From fundamental science to clinical applications[J]. Nat Rev Mol Cell Biol, 2021, 22(9): 608-624. DOI: 10.1038/s41580-021-00373-7. |
| [27] |
BARBAY V, HOUSSARI M, MEKKI M, et al. Role of M2-like macrophage recruitment during angiogenic growth factor therapy[J]. Angiogenesis, 2015, 18(2): 191-200. DOI: 10.1007/s10456-014-9456-z. |
| [28] |
DONG X, LIU J, XU Y, et al. Role of macrophages in experimental liver injury and repair in mice[J]. Exp Ther Med, 2019, 17(5): 3835-3847. DOI: 10.3892/etm.2019.7450. |








 DownLoad:
DownLoad: