| [1] |
General Office of National Health Commission. Standard for diagnosis and treatment of pancreatic cancer (2022 edition)[J]. J Clin Hepatol, 2022, 38(5): 1006-1030. DOI: 10.3969/j.issn.1001-5256.2022.05.007. |
| [2] |
BRAY F, FERLAY J, SOERJOMATARAM I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA Cancer J Clin, 2018, 68(6): 394-424. DOI: 10.3322/caac.21492. |
| [3] |
SIEGEL RL, MILLER KD, JEMAL A. Cancer statistics, 2020[J]. CA Cancer J Clin, 2020, 70(1): 7-30. DOI: 10.3322/caac.21590. |
| [4] |
TANG D, WU Q, ZHANG J, et al. Galectin-1 expression in activated pancreatic satellite cells promotes fibrosis in chronic pancreatitis/pancreatic cancer via the TGF-β1/Smad pathway[J]. Oncol Rep, 2018, 39(3): 1347-1355. DOI: 10.3892/or.2018.6202. |
| [5] |
KAJDANIUK D, MAREK B, BORGIEL-MAREK H, et al. Transforming growth factor β1 (TGFβ1) in physiology and pathology[J]. Endokrynol Pol, 2013, 64(5): 384-396. DOI: 10.5603/EP.2013.0022. |
| [6] |
KUMARI A, SHONIBARE Z, MONAVARIAN M, et al. TGFβ signaling networks in ovarian cancer progression and plasticity[J]. Clin Exp Metastasis, 2021, 38(2): 139-161. DOI: 10.1007/s10585-021-10077-z. |
| [7] |
GALLO-OLLER G, DI SCALA M, ARANDA F, et al. Transforming growth factor beta (TGF-β) activity in immuno-oncology studies[J]. Methods Enzymol, 2020, 636: 129-172. DOI: 10.1016/bs.mie.2019.06.008. |
| [8] |
ZHAO Y, QIAO W, WANG X, et al. 14-3-3ζ/TGFβR1 promotes tumor metastasis in lung squamous cell carcinoma[J]. Oncotarget, 2016, 7(50): 82972-82984. DOI: 10.18632/oncotarget.12690. |
| [9] |
FAN JY, WANG J. Expression of Mir-196b and TGFβR1 in pancreatic ductal adenocarcinoma and its significance[J]. Shandong Med J, 2021, 61(10): 53-56. DOI: 10.3969/j.issn.1002-266X. |
| [10] |
PRINCIPE DR, DOLL JA, BAUER J, et al. TGF-β: duality of function between tumor prevention and carcinogenesis[J]. J Natl Cancer Inst, 2014, 106(2): djt369. DOI: 10.1093/jnci/djt369. |
| [11] |
WEISS A, ATTISANO L. The TGFbeta superfamily signaling pathway[J]. Wiley Interdiscip Rev Dev Biol, 2013, 2(1): 47-63. DOI: 10.1002/wdev.86. |
| [12] |
JIA EC, ZHU WL. Research progress of transforming growth factor β Ⅱ receptor in digestive system tumors. [J]. Chongqing Med J, 2016, 45(23): 3293-3295, 3299. DOI: 10.3969/j.issn.1671-8348.2016.23.046. |
| [13] |
GUO W, DONG Z, GUO Y, et al. Association of polymorphisms in transforming growth factor-β receptors with susceptibility to gastric cardia adenocarcinoma[J]. Mol Biol Rep, 2012, 39(4): 4301-4309. DOI: 10.1007/s11033-011-1217-0. |
| [14] |
BIANKIN AV, WADDELL N, KASSAHN KS, et al. Pancreatic cancer genomes reveal aberrations in axon guidance pathway genes[J]. Nature, 2012, 491(7424): 399-405. DOI: 10.1038/nature11547. |
| [15] |
CHEN YN, ZHANG XY. Research progress on the role of transforming growth factor β Ⅲ receptor in the development of human tumors[J]. J Modern Oncol, 2022, 30(10): 1907-1910. DOI: 10.3969/j.issn.1672-4992. |
| [16] |
|
| [17] |
YIN Z, MA T, HUANG B, et al. Macrophage-derived exosomal microRNA-501-3p promotes progression of pancreatic ductal adenocarcinoma through the TGFBR3-mediated TGF-β signaling pathway[J]. J Exp Clin Cancer Res, 2019, 38(1): 310. DOI: 10.1186/s13046-019-1313-x. |
| [18] |
ZOU ML, CHEN ZH, TENG YY, et al. The Smad dependent TGF-β and BMP signaling pathway in bone remodeling and therapies[J]. Front Mol Biosci, 2021, 8: 593310. DOI: 10.3389/fmolb.2021.593310. |
| [19] |
MARTIN-MALPARTIDA P, BATET M, KACZMARSKA Z, et al. Structural basis for genome wide recognition of 5-bp GC motifs by SMAD transcription factors[J]. Nat Commun, 2017, 8(1): 2070. DOI: 10.1038/s41467-017-02054-6. |
| [20] |
PRINCIPE DR, DECANT B, MASCARIÑAS E, et al. TGFβ signaling in the pancreatic tumor microenvironment promotes fibrosis and immune evasion to facilitate tumorigenesis[J]. Cancer Res, 2016, 76(9): 2525-2539. DOI: 10.1158/0008-5472.CAN-15-1293. |
| [21] |
WANG F, XIA X, YANG C, et al. SMAD4 gene mutation renders pancreatic cancer resistance to radiotherapy through promotion of autophagy[J]. Clin Cancer Res, 2018, 24(13): 3176-3185. DOI: 10.1158/1078-0432.CCR-17-3435. |
| [22] |
SHUGANG X, HONGFA Y, JIANPENG L, et al. Prognostic value of SMAD4 in pancreatic cancer: A meta-analysis[J]. Transl Oncol, 2016, 9(1): 1-7. DOI: 10.1016/j.tranon.2015.11.007. |
| [23] |
DU Y, ZHOU X, HUANG Z, et al. Meta-analysis of the prognostic value of smad4 immunohistochemistry in various cancers[J]. PLoS One, 2014, 9(10): e110182. DOI: 10.1371/journal.pone.0110182. |
| [24] |
WANG JD, JIN K, CHEN XY, et al. Clinicopathological significance of SMAD4 loss in pancreatic ductal adenocarcinomas: a systematic review and meta-analysis[J]. Oncotarget, 2017, 8(10): 16704-16711. DOI: 10.18632/oncotarget.14335. |
| [25] |
KANG Y, LING J, SUZUKI R, et al. SMAD4 regulates cell motility through transcription of N-cadherin in human pancreatic ductal epithelium[J]. PLoS One, 2014, 9(9): e107948. DOI: 10.1371/journal.pone.0107948. |
| [26] |
LEUNG L, RADULOVICH N, ZHU CQ, et al. Loss of canonical Smad4 signaling promotes KRAS driven malignant transformation of human pancreatic duct epithelial cells and metastasis[J]. PLoS One, 2013, 8(12): e84366. DOI: 10.1371/journal.pone.0084366. |
| [27] |
LI Y, BASTI A, YALÇIN M, et al. Circadian dysregulation of the TGFβ/SMAD4 pathway modulates metastatic properties and cell fate decisions in pancreatic cancer cells[J]. iScience, 2020, 23(10): 101551. DOI: 10.1016/j.isci.2020.101551. |
| [28] |
DARDARE J, WITZ A, MERLIN JL, et al. SMAD4 and the TGFβ pathway in patients with pancreatic ductal adenocarcinoma[J]. Int J Mol Sci, 2020, 21(10). DOI: 10.3390/ijms21103534. |
| [29] |
NEUZILLET C, HAMMEL P, TIJERAS-RABALLAND A, et al. Targeting the Ras-ERK pathway in pancreatic adenocarcinoma[J]. Cancer Metastasis Rev, 2013, 32(1-2): 147-162. DOI: 10.1007/s10555-012-9396-2. |
| [30] |
BUONATO JM, LAZZARA MJ. ERK1/2 blockade prevents epithelial-mesenchymal transition in lung cancer cells and promotes their sensitivity to EGFR inhibition[J]. Cancer Res, 2014, 74(1): 309-319. DOI: 10.1158/0008-5472.CAN-12-4721. |
| [31] |
PRINCIPE DR, DIAZ AM, TORRES C, et al. TGFβ engages MEK/ERK to differentially regulate benign and malignant pancreas cell function[J]. Oncogene, 2017, 36(30): 4336-4348. DOI: 10.1038/onc.2016.500. |
| [32] |
GUO Q. Changes in mitochondrial function during EMT induced by TGFβ-1 in pancreatic cancer[J]. Oncol Lett, 2017, 13(3): 1575-1580. DOI: 10.3892/ol.2017.5613. |
| [33] |
CHANG CH, PAUKLIN S. ROS and TGFβ: from pancreatic tumour growth to metastasis[J]. J Exp Clin Cancer Res, 2021, 40(1): 152. DOI: 10.1186/s13046-021-01960-4. |
| [34] |
SANJABI S, OH SA, LI MO. Regulation of the immune response by TGF-β: From conception to autoimmunity and infection[J]. Cold Spring Harb Perspect Biol, 2017, 9(6): a022236. DOI: 10.1101/cshperspect.a022236. |
| [35] |
MA B, LI JG, WANG J, et al. Promotion effect of miR-106b on invasion and migration of colon cancer cells through targeting TGF-β/Smad pathway[J]. J Jilin Univ(Med Edit), 2021, 47(3): 636-636. DOI: 10.13481/j.1671-587Ⅹ.20210312. |
| [36] |
DAVID CJ, MASSAGUÉ J. Contextual determinants of TGFβ action in development, immunity and cancer[J]. Nat Rev Mol Cell Biol, 2018, 19(7): 419-435. DOI: 10.1038/s41580-018-0007-0. |
| [37] |
ANDERTON MJ, MELLOR HR, BELL A, et al. Induction of heart valve lesions by small-molecule ALK5 inhibitors[J]. Toxicol Pathol, 2011, 39(6): 916-924. DOI: 10.1177/0192623311416259. |
| [38] |
MELISI D, GARCIA-CARBONERO R, MACARULLA T, et al. Galunisertib plus gemcitabine vs. gemcitabine for first-line treatment of patients with unresectable pancreatic cancer[J]. Br J Cancer, 2018, 119(10): 1208-1214. DOI: 10.1038/s41416-018-0246-z. |
| [39] |
FAIVRE S, SANTORO A, KELLEY RK, et al. Novel transforming growth factor beta receptor I kinase inhibitor galunisertib (LY2157299) in advanced hepatocellular carcinoma[J]. Liver Int, 2019, 39(8): 1468-1477. DOI: 10.1111/liv.14113. |
| [40] |
KOVACS RJ, MALDONADO G, AZARO A, et al. Cardiac safety of TGF-β receptor i kinase inhibitor LY2157299 monohydrate in cancer patients in a First-in-Human dose study[J]. Cardiovasc Toxicol, 2015, 15(4): 309-323. DOI: 10.1007/s12012-014-9297-4. |



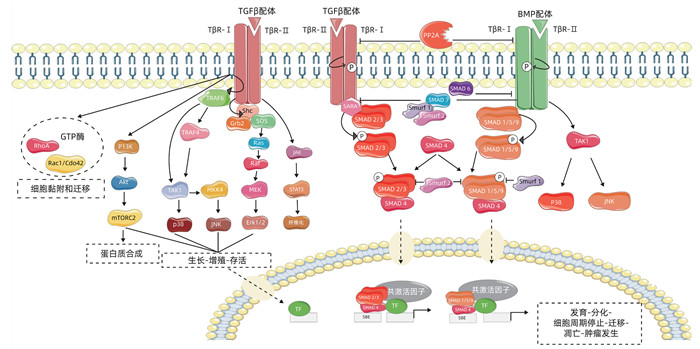




 DownLoad:
DownLoad: