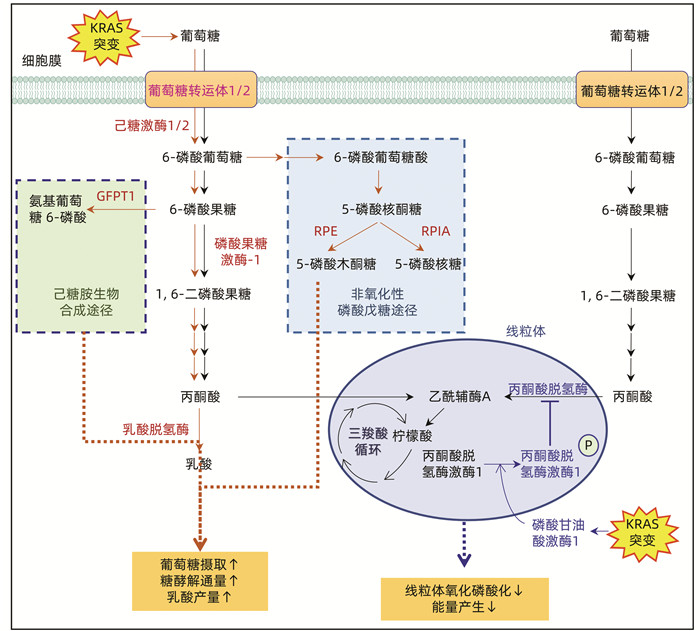
| [1] |
SIEGEL RL, MILLER KD, FUCHS HE, et al. Cancer statistics, 2021[J]. CA Cancer J Clin, 2021, 71(1): 7-33. DOI: 10.3322/caac.21654. |
| [2] |
BRAY F, FERLAY J, SOERJOMATARAM I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA Cancer J Clin, 2018, 68(6): 394-424. DOI: 10.3322/caac.21492. |
| [3] |
HUANG L, JANSEN L, BALAVARCA Y, et al. Resection of pancreatic cancer in Europe and USA: an international large-scale study highlighting large variations[J]. Gut, 2019, 68(1): 130-139. DOI: 10.1136/gutjnl-2017-314828. |
| [4] |
HENRIKSEN A, DYHL-POLK A, CHEN I, et al. Checkpoint inhibitors in pancreatic cancer[J]. Cancer Treat Rev, 2019, 78: 17-30. DOI: 10.1016/j.ctrv.2019.06.005. |
| [5] |
INDINI A, RIJAVEC E, GHIDINI M, et al. Targeting KRAS in solid tumors: Current challenges and future opportunities of novel KRAS inhibitors[J]. Pharmaceutics, 2021, 13(5): 653. DOI: 10.3390/pharmaceutics13050653. |
| [6] |
HOSEIN AN, BREKKEN RA, MAITRA A. Pancreatic cancer stroma: an update on therapeutic targeting strategies[J]. Nat Rev Gastroenterol Hepatol, 2020, 17(8): 487-505. DOI: 10.1038/s41575-020-0300-1. |
| [7] |
SUN H, ZHANG B, LI H. The Roles of frequently mutated genes of pancreatic cancer in regulation of tumor microenvironment[J]. Technol Cancer Res Treat, 2020, 19: 1533033820920969. DOI: 10.1177/1533033820920969. |
| [8] |
HOLLINSHEAD K, PARKER SJ, EAPEN VV, et al. Respiratory supercomplexes promote mitochondrial efficiency and growth in severely hypoxic pancreatic cancer[J]. Cell Rep, 2020, 33(1): 108231. DOI: 10.1016/j.celrep.2020.108231. |
| [9] |
DEY P, LI J, ZHANG J, et al. Oncogenic KRAS-Driven metabolic reprogramming in pancreatic cancer cells utilizes cytokines from the tumor microenvironment[J]. Cancer Discov, 2020, 10(4): 608-625. DOI: 10.1158/2159-8290.CD-19-0297. |
| [10] |
BUSCAIL L, BOURNET B, CORDELIER P. Role of oncogenic KRAS in the diagnosis, prognosis and treatment of pancreatic cancer[J]. Nat Rev Gastroenterol Hepatol, 2020, 17(3): 153-168. DOI: 10.1038/s41575-019-0245-4. |
| [11] |
ENCARNACIÓN-ROSADO J, KIMMELMAN AC. Harnessing metabolic dependencies in pancreatic cancers[J]. Nat Rev Gastroenterol Hepatol, 2021, 18(7): 482-492. DOI: 10.1038/s41575-021-00431-7. |
| [12] |
NAGDAS S, KASHATUS JA, NASCIMENTO A, et al. Drp1 promotes KRas-driven metabolic changes to drive pancreatic tumor growth[J]. Cell Rep, 2019, 28(7): 1845-1859.e5. DOI: 10.1016/j.celrep.2019.07.031. |
| [13] |
HOU P, KAPOOR A, ZHANG Q, et al. Tumor microenvironment remodeling enables bypass of oncogenic KRAS dependency in pancreatic cancer[J]. Cancer Discov, 2020, 10(7): 1058-1077. DOI: 10.1158/2159-8290.CD-19-0597. |
| [14] |
SANTANA-CODINA N, ROETH AA, ZHANG Y, et al. Oncogenic KRAS supports pancreatic cancer through regulation of nucleotide synthesis[J]. Nat Commun, 2018, 9(1): 4945. DOI: 10.1038/s41467-018-07472-8. |
| [15] |
LI X, JIANG Y, MEISENHELDER J, et al. Mitochondria-translocated PGK1 functions as a protein kinase to coordinate glycolysis and the TCA cycle in tumorigenesis[J]. Mol Cell, 2016, 61(5): 705-719. DOI: 10.1016/j.molcel.2016.02.009. |
| [16] |
ROZEVELD CN, JOHNSON KM, ZHANG L, et al. KRAS controls pancreatic cancer cell lipid metabolism and invasive potential through the lipase HSL[J]. Cancer Res, 2020, 80(22): 4932-4945. DOI: 10.1158/0008-5472.CAN-20-1255. |
| [17] |
ROSSI SEBASTIANO M, POZZATO C, SALIAKOURA M, et al. ACSL3-PAI-1 signaling axis mediates tumor-stroma cross-talk promoting pancreatic cancer progression[J]. Sci Adv, 2020, 6(44): eabb9200. DOI: 10.1126/sciadv.abb9200. |
| [18] |
ROSSI SEBASTIANO M, KONSTANTINIDOU G. Targeting long chain Acyl-CoA synthetases for cancer therapy[J]. Int J Mol Sci, 2019, 20(15): 3624. DOI: 10.3390/ijms20153624. |
| [19] |
SALIAKOURA M, SEBASTIANO MR, NIKDIMA I, et al. Restriction of extracellular lipids renders pancreatic cancer dependent on autophagy[J]. J Exp Clin Cancer Res, 2022, 41(1): 16. DOI: 10.1186/s13046-021-02231-y. |
| [20] |
WANG YP, ZHOU W, WANG J, et al. Arginine methylation of MDH1 by CARM1 inhibits glutamine metabolism and suppresses pancreatic cancer[J]. Mol Cell, 2016, 64(4): 673-687. DOI: 10.1016/j.molcel.2016.09.028. |
| [21] |
RAHO S, CAPOBIANCO L, MALIVINDI R, et al. KRAS-regulated glutamine metabolism requires UCP2-mediated aspartate transport to support pancreatic cancer growth[J]. Nat Metab, 2020, 2(12): 1373-1381. DOI: 10.1038/s42255-020-00315-1. |
| [22] |
ZHANG Y, COMMISSO C. Macropinocytosis in cancer: A complex signaling network[J]. Trends Cancer, 2019, 5(6): 332-334. DOI: 10.1016/j.trecan.2019.04.002. |
| [23] |
RAMIREZ C, HAUSER AD, VUCIC EA, et al. Plasma membrane V-ATPase controls oncogenic RAS-induced macropinocytosis[J]. Nature, 2019, 576(7787): 477-481. DOI: 10.1038/s41586-019-1831-x. |
| [24] |
KINSEY CG, CAMOLOTTO SA, BOESPFLUG AM, et al. Publisher correction: Protective autophagy elicited by RAF→MEK→ERK inhibition suggests a treatment strategy for RAS-driven cancers[J]. Nat Med, 2019, 25(5): 861. DOI: 10.1038/s41591-019-0433-3. |
| [25] |
HUMPTON TJ, ALAGESAN B, DENICOLA GM, et al. Oncogenic KRAS induces NIX-mediated mitophagy to promote pancreatic cancer[J]. Cancer Discov, 2019, 9(9): 1268-1287. DOI: 10.1158/2159-8290.CD-18-1409. |
| [26] |
YAMAMOTO K, VENIDA A, YANO J, et al. Autophagy promotes immune evasion of pancreatic cancer by degrading MHC-I[J]. Nature, 2020, 581(7806): 100-105. DOI: 10.1038/s41586-020-2229-5. |
| [27] |
POMMIER A, ANAPARTHY N, MEMOS N, et al. Unresolved endoplasmic reticulum stress engenders immune-resistant, latent pancreatic cancer metastases[J]. Science, 2018, 360(6394). DOI: 10.1126/science.aao4908. |
| [28] |
DAI E, HAN L, LIU J, et al. Autophagy-dependent ferroptosis drives tumor-associated macrophage polarization via release and uptake of oncogenic KRAS protein[J]. Autophagy, 2020, 16(11): 2069-2083. DOI: 10.1080/15548627.2020.1714209. |
| [29] |
MONTERAN L, EREZ N. The dark side of fibroblasts: Cancer-associated fibroblasts as mediators of immunosuppression in the tumor microenvironment[J]. Front Immunol, 2019, 10: 1835. DOI: 10.3389/fimmu.2019.01835. |
| [30] |
HAMARSHEH S, GROβ O, BRUMMER T, et al. Immune modulatory effects of oncogenic KRAS in cancer[J]. Nat Commun, 2020, 11(1): 5439. DOI: 10.1038/s41467-020-19288-6. |
| [31] |
WANG T, NOTTA F, NAVAB R, et al. Senescent carcinoma-associated fibroblasts upregulate IL8 to enhance prometastatic phenotypes[J]. Mol Cancer Res, 2017, 15(1): 3-14. DOI: 10.1158/1541-7786.MCR-16-0192. |
| [32] |
HAFEZI S, SABER-AYAD M, ABDEL-RAHMAN WM. Highlights on the role of KRAS mutations in reshaping the microenvironment of pancreatic adenocarcinoma[J]. Int J Mol Sci, 2021, 22(19): 10219. DOI: 10.3390/ijms221910219. |
| [33] |
NOLLMANN FI, RUESS DA. Targeting mutant KRAS in pancreatic cancer: Futile or promising?[J]. Biomedicines, 2020, 8(8): 281. DOI: 10.3390/biomedicines8080281. |
| [34] |
CANON J, REX K, SAIKI AY, et al. The clinical KRAS(G12C) inhibitor AMG 510 drives anti-tumour immunity[J]. Nature, 2019, 575(7781): 217-223. DOI: 10.1038/s41586-019-1694-1. |
| [35] |
HALLIN J, ENGSTROM LD, HARGIS L, et al. The KRASG12C inhibitor MRTX849 provides insight toward therapeutic susceptibility of KRAS-mutant cancers in mouse models and patients[J]. Cancer Discov, 2020, 10(1): 54-71. DOI: 10.1158/2159-8290.CD-19-1167. |
| [36] |
HONG DS, FAKIH MG, STRICKLER JH, et al. KRASG12C inhibition with sotorasib in advanced solid tumors[J]. N Engl J Med, 2020, 383(13): 1207-1217. DOI: 10.1056/NEJMoa1917239. |
| [37] |
JONCKHEERE N, VASSEUR R, VAN SEUNINGEN I. The cornerstone K-RAS mutation in pancreatic adenocarcinoma: From cell signaling network, target genes, biological processes to therapeutic targeting[J]. Crit Rev Oncol Hematol, 2017, 111: 7-19. DOI: 10.1016/j.critrevonc.2017.01.002. |
| [38] |
DROSTEN M, BARBACID M. Targeting the MAPK pathway in KRAS-driven tumors[J]. Cancer Cell, 2020, 37(4): 543-550. DOI: 10.1016/j.ccell.2020.03.013. |
| [39] |
TERRELL EM, MORRISON DK. Ras-mediated activation of the raf family kinases[J]. Cold Spring Harb Perspect Med, 2019, 9(1): a033746. DOI: 10.1101/cshperspect.a033746. |
| [40] |
NAGASAKA M, POTUGARI B, NGUYEN A, et al. KRAS Inhibitors- yes but what next? Direct targeting of KRAS-vaccines, adoptive T cell therapy and beyond[J]. Cancer Treat Rev, 2021, 101: 102309. DOI: 10.1016/j.ctrv.2021.102309. |



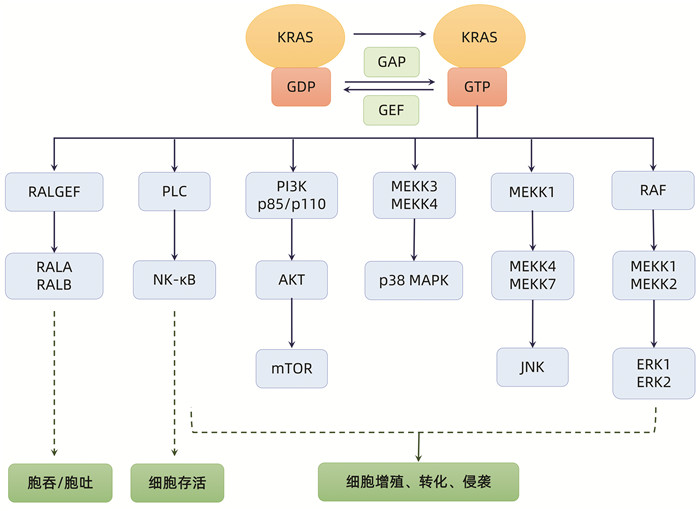




 DownLoad:
DownLoad: