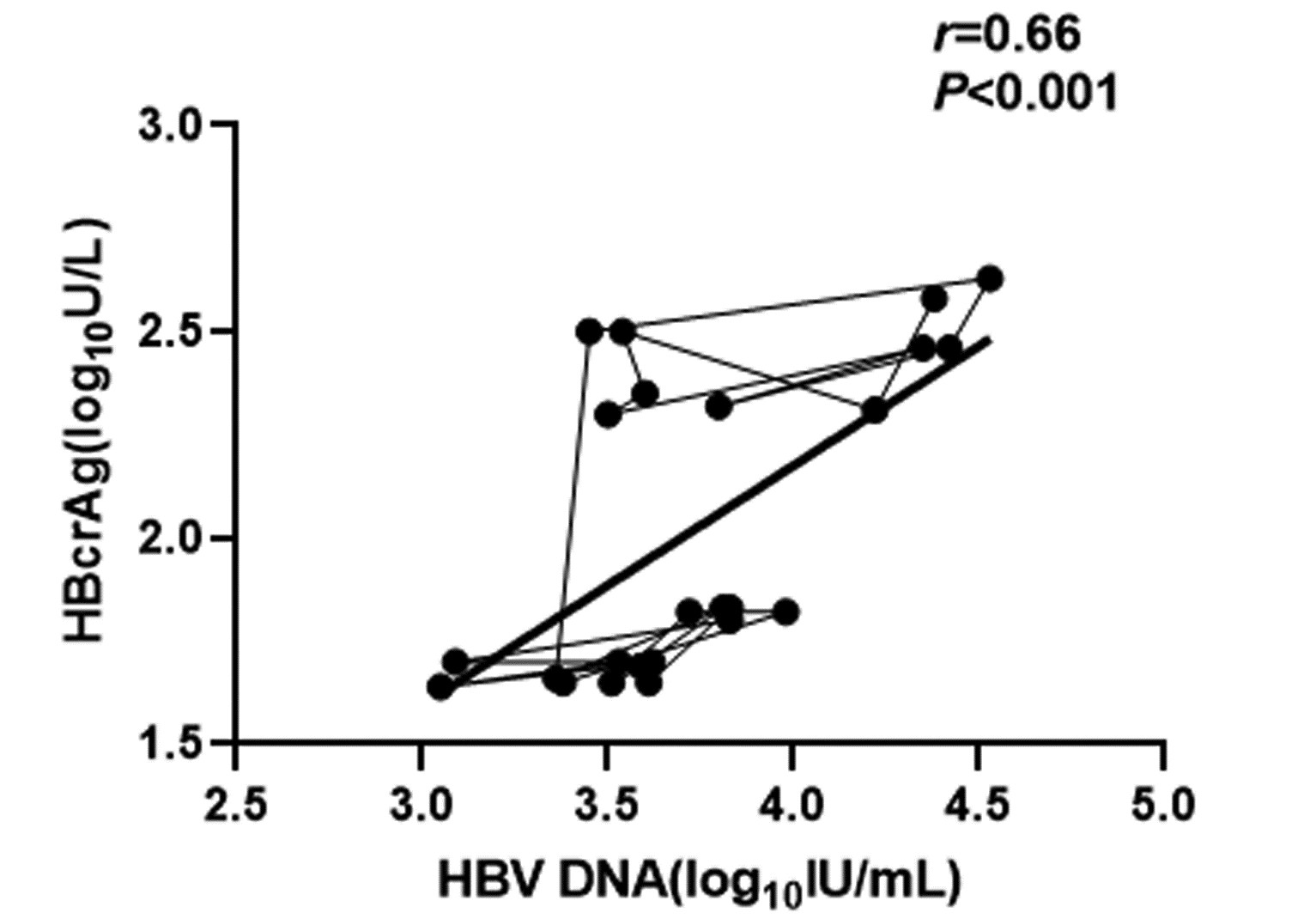
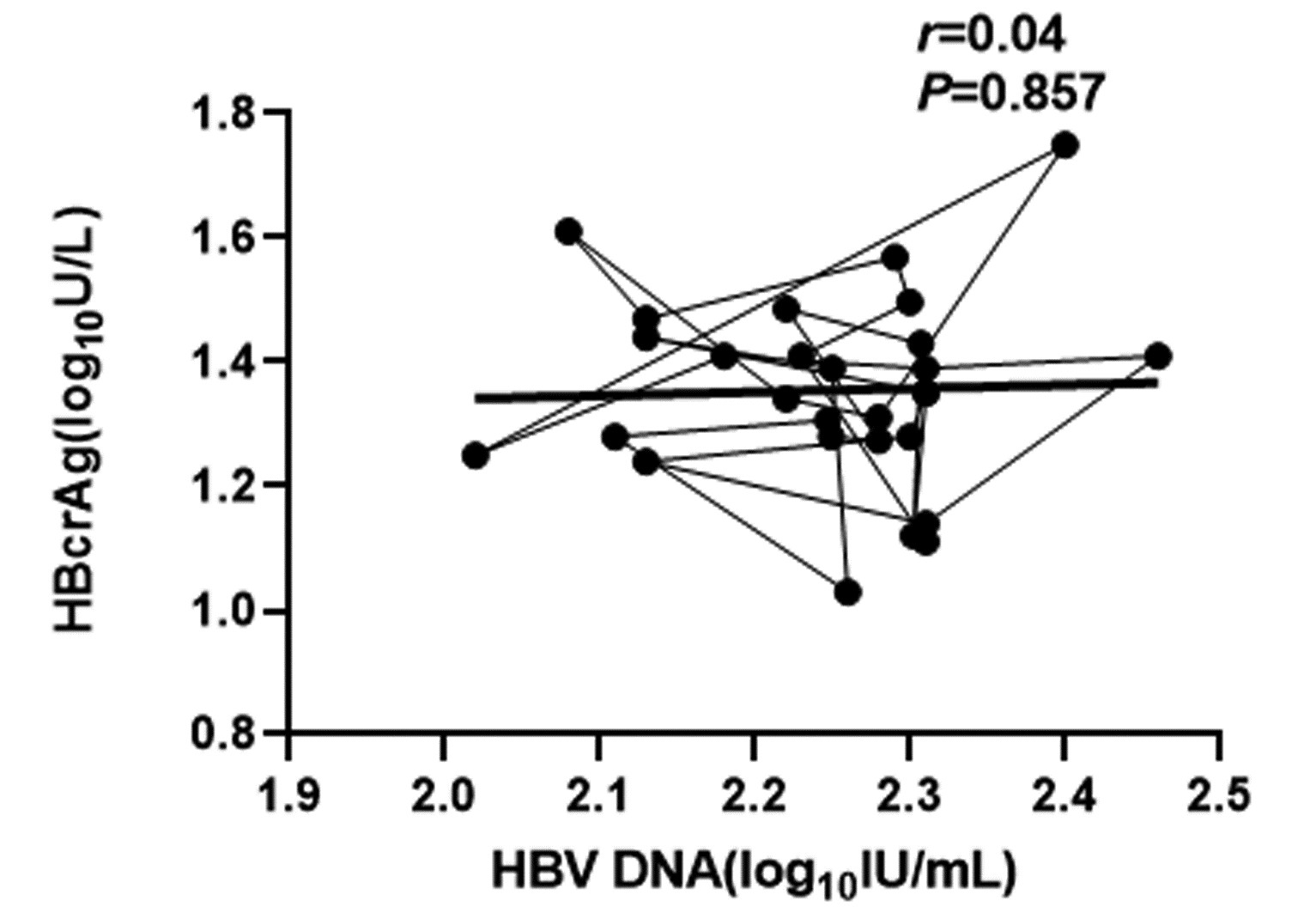
| [1] |
TANG L, COVERT E, WILSON E, et al. Chronic hepatitis B infection: a review[J]. JAMA, 2018, 319(17): 1802-1813. DOI: 10.1001/jama.2018.3795. |
| [2] |
BAI L, ZHANG X, KOZLOWSKI M, et al. Extracellular hepatitis B virus RNAs are heterogeneous in length and circulate as capsid-antibody complexes in addition to virions in chronic hepatitis B patients[J]. J Virol, 2018, 92(24): e00798-18. DOI: 10.1128/JVI.00798-18. |
| [3] |
NING X, LUCKENBAUGH L, LIU K, et al. Common and distinct capsid and surface protein requirements for secretion of complete and genome-free hepatitis B virions[J]. J Virol, 2018, 92(14): e00272-18. DOI: 10.1128/JVI.00272-18. |
| [4] |
LU L, ZHANG HY, YUENG YH, et al. Intracellular levels of hepatitis B virus DNA and pregenomic RNA in peripheral blood mononuclear cells of chronically infected patients[J]. J Viral Hepat, 2009, 16(2): 104-112. DOI: 10.1111/j.1365-2893.2008.01054.x. |
| [5] |
LARSSON SB, MALMSTRÖM S, HANNOUN C, et al. Mechanisms downstream of reverse transcription reduce serum levels of HBV DNA but not of HBsAg in chronic hepatitis B virus infection[J]. Virol J, 2015, 12: 213. DOI: 10.1186/s12985-015-0447-5. |
| [6] |
|
| [7] |
WANG XR, HU XJ, WANG XR, et al. Correlation between the expressions of HBV pgRNA and HBV-LP in serum and the degree of liver fibrosis in patients with chronic HBV infection[J]. Int J Virol, 2021, 28(2): 149-153. DOI: 10.3760/cma.j.issn.1673-4092.2021.02.014. |
| [8] |
CHEN LY, QI CX, AN WN, et al. Levels and predictive value of HBcrAg in CHB patients with HBeAg positive in treatment with entecavir[J/CD]. Chin J Liver Dis (Electronic Version), 2022, 14(2): 50-56. DOI: 10.3969/j.issn.1674-7380.2022.02.008. |
| [9] |
European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection[J]. J Hepatol, 2017, 67(2): 370-398. DOI: 10.1016/j.jhep.2017.03.021. |
| [10] |
|
| [11] |
NASSAL M. HBV cccDNA: viral persistence reservoir and key obstacle for a cure of chronic hepatitis B[J]. Gut, 2015, 64(12): 1972-1984. DOI: 10.1136/gutjnl-2015-309809. |
| [12] |
CHEN EQ, FENG S, WANG ML, et al. Serum hepatitis B core-related antigen is a satisfactory surrogate marker of intrahepatic covalently closed circular DNA in chronic hepatitis B[J]. Sci Rep, 2017, 7(1): 173. DOI: 10.1038/s41598-017-00111-0. |
| [13] |
WANG J, SHEN T, HUANG X, et al. Serum hepatitis B virus RNA is encapsidated pregenome RNA that may be associated with persistence of viral infection and rebound[J]. J Hepatol, 2016, 65(4): 700-710. DOI: 10.1016/j.jhep.2016.05.029. |
| [14] |
LING XZ, WANG RM, SU MH, et al. Clinical significance of dynamic monitoring of serum HBV pgRNA in CHB patients treated with Nas[J]. Chin J Pract Intern Med, 2022, 42(5): 404-408. DOI: 10.19538/j.nk2022050112. 零小樟, 王荣明, 苏明华, 等. 慢性乙型肝炎患者核苷酸类似物治疗中动态监测血清乙型肝炎病毒前基因组RNA的临床意义[J]. 中国实用内科杂志, 2022, 42(5): 404-408. DOI: 10.19538/j.nk2022050112. |
| [15] |
HÖNER ZU SIEDERDISSEN C, RINKER F, MAASOUMY B, et al. Viral and host responses after stopping long-term nucleos(t)ide analogue therapy in HBeAg-negative chronic hepatitis B[J]. J Infect Dis, 2016, 214(10): 1492-1497. DOI: 10.1093/infdis/jiw412. |
| [16] |
ZIMMER CL, RINKER F, HÖNER ZU SIEDERDISSEN C, et al. Increased NK cell function after cessation of long-term nucleos(t)ide analogue treatment in chronic hepatitis B is associated with liver damage and HBsAg loss[J]. J Infect Dis, 2018, 217(10): 1656-1666. DOI: 10.1093/infdis/jiy097. |
| [17] |
LIAO H, LIU Y, LI X, et al. Monitoring of serum HBV RNA, HBcrAg, HBsAg and anti-HBc levels in patients during long-term nucleoside/nucleotide analogue therapy[J]. Antivir Ther, 2019, 24(2): 105-115. DOI: 10.3851/IMP3280. |
| [18] |
BUTLER EK, GERSCH J, MCNAMARA A, et al. Hepatitis B virus serum DNA and RNA levels in nucleos(t)ide analog-treated or untreated patients during chronic and acute infection[J]. Hepatology, 2018, 68(6): 2106-2117. DOI: 10.1002/hep.30082. |



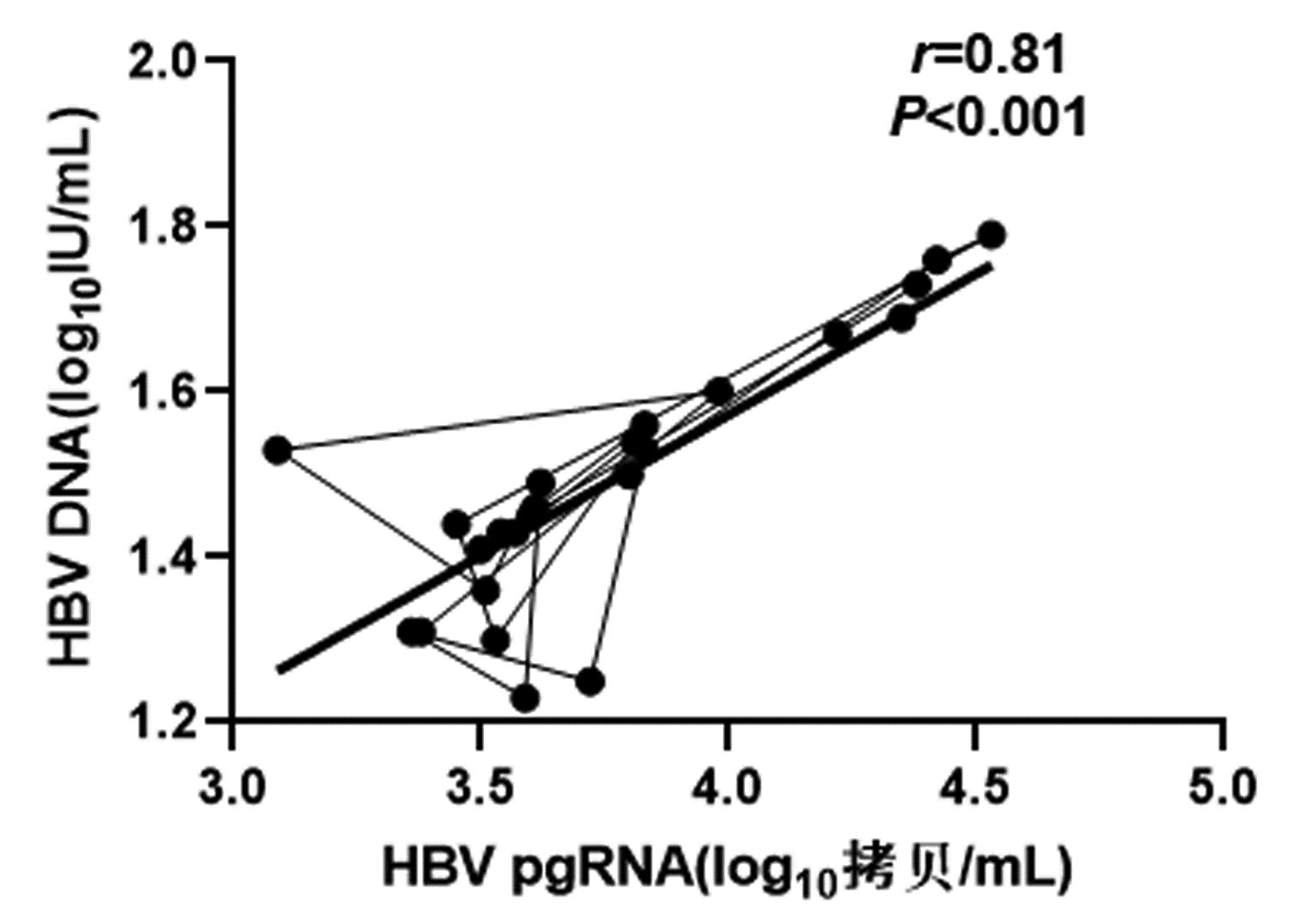




 DownLoad:
DownLoad: