| [1] |
GBD 2013 Mortality and Causes of Death Collaborators. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013[J]. Lancet, 2015, 385(9963): 117-171. DOI: 10.1016/S0140-6736(14)61682-2. |
| [2] |
Chinese Society of Infectious Diseases, Chinese Medical Association; Chinese Society of Hepatology, Chinese Medical Association. Guidelines for the prevention and treatment of chronic hepatitis B (version 2019)[J]. J Clin Hepatol, 2019, 35(12): 2648-2669. DOI: 10.3969/j.issn.1001-5256.2019.12.007. |
| [3] |
|
| [4] |
XIA Y, CHENG X, LI Y, et al. Hepatitis B virus deregulates the cell cycle to promote viral replication and a premalignant phenotype[J]. J Virol, 2018, 92(19): e00722-18. DOI: 10.1128/JVI.00722-18. |
| [5] |
YANG Y, YAN Y, CHEN Z, et al. Histone deacetylase inhibitors romidepsin and vorinostat promote hepatitis B virus replication by inducing cell cycle arrest[J]. J Clin Transl Hepatol, 2021, 9(2): 160-168. DOI: 10.14218/JCTH.2020.00105. |
| [6] |
OTTO T, SICINSKI P. Cell cycle proteins as promising targets in cancer therapy[J]. Nat Rev Cancer, 2017, 17(2): 93-115. DOI: 10.1038/nrc.2016.138. |
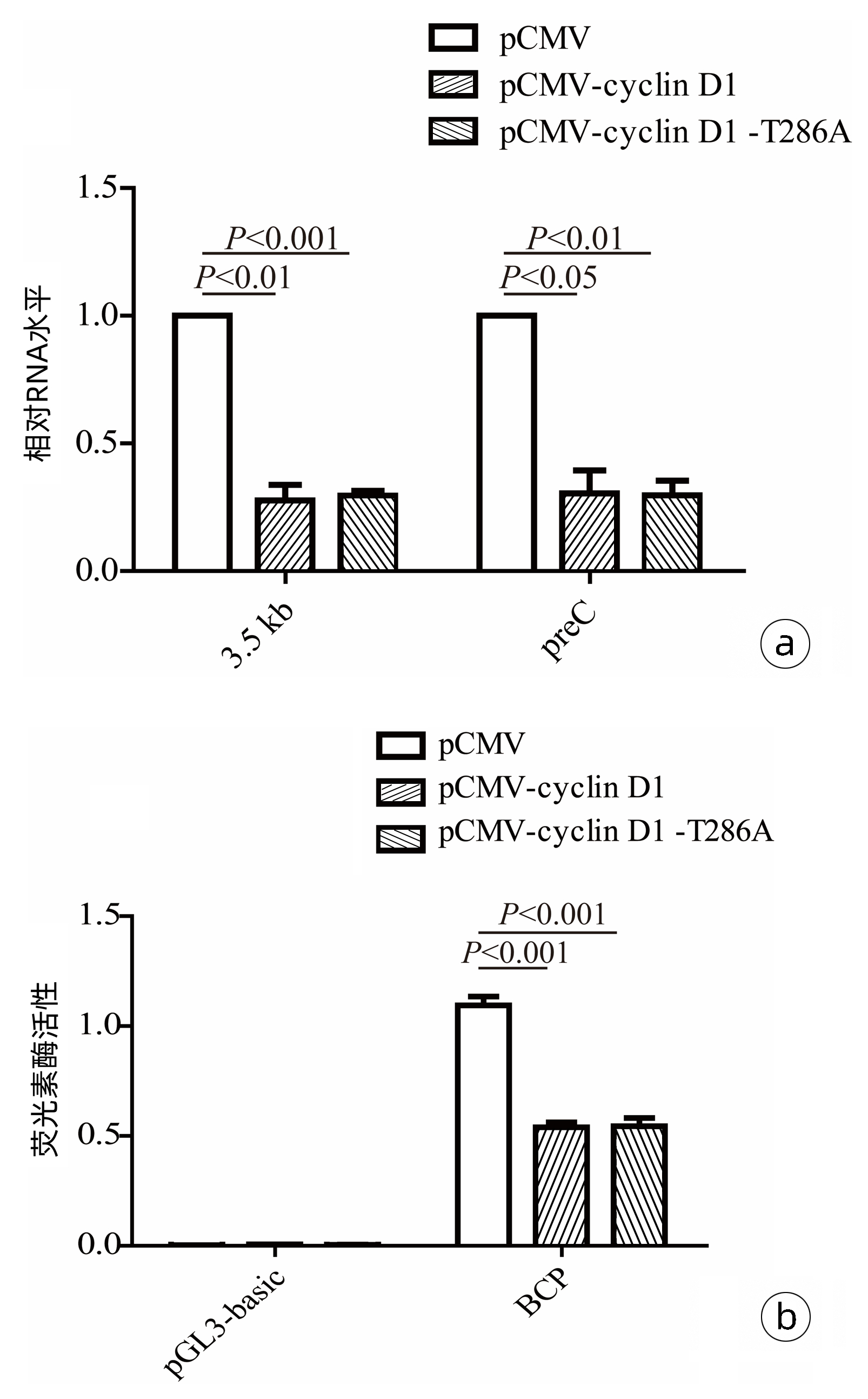
| [7] |
|
| [8] |
QI Z, LI G, HU H, et al. Recombinant covalently closed circular hepatitis B virus DNA induces prolonged viral persistence in immunocompetent mice[J]. J Virol, 2014, 88(14): 8045-8056. DOI: 10.1128/JVI.01024-14. |
| [9] |
WANG M, GONG Q, ZHANG J, et al. Characterization of gene expression profiles in HBV-related liver fibrosis patients and identification of ITGBL1 as a key regulator of fibrogenesis[J]. Sci Rep, 2017, 7: 43446. DOI: 10.1038/srep43446. |
| [10] |
ZHOU W, MA Y, ZHANG J, et al. Predictive model for inflammation grades of chronic hepatitis B: Large-scale analysis of clinical parameters and gene expressions[J]. Liver Int, 2017, 37(11): 1632-1641. DOI: 10.1111/liv.13427. |
| [11] |
RITCHIE ME, PHIPSON B, WU D, et al. limma powers differential expression analyses for RNA-sequencing and microarray studies[J]. Nucleic Acids Res, 2015, 43(7): e47. DOI: 10.1093/nar/gkv007. |
| [12] |
R Core Team (2022). R: A language and environment for statistical computing[EB/OL]. https://www.R-project.org/.
|
| [13] |
KANG J, WANG J, CHENG J, et al. Down-regulation of NTCP expression by cyclin D1 in hepatitis B virus-related hepatocellular carcinoma has clinical significance[J]. Oncotarget, 2017, 8(34): 56041-56050. DOI: 10.18632/oncotarget.10241. |
| [14] |
JUNG YJ, KIM JW, PARK SJ, et al. c-Myc-mediated overexpression of miR-17-92 suppresses replication of hepatitis B virus in human hepatoma cells[J]. J Med Virol, 2013, 85(6): 969-978. DOI: 10.1002/jmv.23534. |
| [15] |
ALMAJHDI FN, AL-QUDARI AY, HUSSAIN Z. Differential expression of transforming growth factor-β1 and HBx enhances hepatitis B virus replication and augments host immune cytokines and chemokines[J]. Ann Hepatol, 2013, 12(3): 408-415.
|
| [16] |
ALT JR, CLEVELAND JL, HANNINK M, et al. Phosphorylation-dependent regulation of cyclin D1 nuclear export and cyclin D1-dependent cellular transformation[J]. Genes Dev, 2000, 14(24): 3102-3114. DOI: 10.1101/gad.854900. |
| [17] |
DIEHL JA, CHENG M, ROUSSEL MF, et al. Glycogen synthase kinase-3beta regulates cyclin D1 proteolysis and subcellular localization[J]. Genes Dev, 1998, 12(22): 3499-3511. DOI: 10.1101/gad.12.22.3499. |
| [18] |
YAN Y, ALLWEISS L, YANG D, et al. Down-regulation of cell membrane localized NTCP expression in proliferating hepatocytes prevents hepatitis B virus infection[J]. Emerg Microbes Infect, 2019, 8(1): 879-894. DOI: 10.1080/22221751.2019.1625728. |
| [19] |
KÖNIG A, YANG J, JO E, et al. Efficient long-term amplification of hepatitis B virus isolates after infection of slow proliferating HepG2-NTCP cells[J]. J Hepatol, 2019, 71(2): 289-300. DOI: 10.1016/j.jhep.2019.04.010. |
| [20] |
ELLER C, HEYDMANN L, COLPITTS CC, et al. A genome-wide gain-of-function screen identifies CDKN2C as a HBV host factor[J]. Nat Commun, 2020, 11(1): 2707. DOI: 10.1038/s41467-020-16517-w. |
| [21] |
RANEY AK, EASTON AJ, MILICH DR, et al. Promoter-specific transactivation of hepatitis B virus transcription by a glutamine- and proline-rich domain of hepatocyte nuclear factor 1[J]. J Virol, 1991, 65(11): 5774-5781. DOI: 10.1128/JVI.65.11.5774-5781.1991. |
| [22] |
ZHENG Y, LI J, OU JH. Regulation of hepatitis B virus core promoter by transcription factors HNF1 and HNF4 and the viral X protein[J]. J Virol, 2004, 78(13): 6908-6914. DOI: 10.1128/JVI.78.13.6908-6914.2004. |
| [23] |
WANG WX, LI M, WU X, et al. HNF1 is critical for the liver-specific function of HBV enhancer Ⅱ[J]. Res Virol, 1998, 149(2): 99-108. DOI: 10.1016/s0923-2516(98)80085-x. |
| [24] |
QUASDORFF M, HÖSEL M, ODENTHAL M, et al. A concerted action of HNF4alpha and HNF1alpha links hepatitis B virus replication to hepatocyte differentiation[J]. Cell Microbiol, 2008, 10(7): 1478-1490. DOI: 10.1111/j.1462-5822.2008.01141.x. |















 DownLoad:
DownLoad: