| [1] |
RIZZETTO M, HAMID S, NEGRO F. The changing context of hepatitis D[J]. J Hepatol, 2021, 74(5): 1200-1211. DOI: 10.1016/j.jhep.2021.01.014. |
| [2] |
PAPATHEODORIDI M, PAPATHEODORIDIS GV. Is hepatitis delta underestimated?[J]. Liver Int, 2021, 41(Suppl 1): 38-44. DOI: 10.1111/liv.14833. |
| [3] |
STOCKDALE AJ, KREUELS B, HENRION M, et al. The global prevalence of hepatitis D virus infection: Systematic review and meta-analysis[J]. J Hepatol, 2020, 73(3): 523-532. DOI: 10.1016/j.jhep.2020.04.008. |
| [4] |
CHEN HY, SHEN DT, JI DZ, et al. Prevalence and burden of hepatitis D virus infection in the global population: a systematic review and meta-analysis[J]. Gut, 2019, 68(3): 512-521. DOI: 10.1136/gutjnl-2018-316601. |
| [5] |
MIAO Z, ZHANG S, OU X, et al. Estimating the global prevalence, disease progression, and clinical outcome of hepatitis delta virus infection[J]. J Infect Dis, 2020, 221(10): 1677-1687. DOI: 10.1093/infdis/jiz633. |
| [6] |
URBAN S, NEUMANN-HAEFELIN C, LAMPERTICO P. Hepatitis D virus in 2021: virology, immunology and new treatment approaches for a difficult-to-treat disease[J]. Gut, 2021, 70(9): 1782-1794. DOI: 10.1136/gutjnl-2020-323888. |
| [7] |
SARIN SK, KUMAR M, LAU GK, et al. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update[J]. Hepatol Int, 2016, 10(1): 1-98. DOI: 10.1007/s12072-015-9675-4. |
| [8] |
European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection[J]. J Hepatol, 2017, 67(2): 370-398. DOI: 10.1016/j.jhep.2017.03.021. |
| [9] |
|
| [10] |
LANGE M, ZARET D, KUSHNER T. Hepatitis delta: Current knowledge and future directions[J]. Gastroenterol Hepatol (N Y), 2022, 18(9): 508-520.
|
| [11] |
DÉNY P. Hepatitis delta virus genetic variability: from genotypes Ⅰ, Ⅱ, Ⅲ to eight major clades?[J]. Curr Top Microbiol Immunol, 2006, 307: 151-171. DOI: 10.1007/3-540-29802-9_8. |
| [12] |
LE GAL F, BRICHLER S, DRUGAN T, et al. Genetic diversity and worldwide distribution of the deltavirus genus: A study of 2, 152 clinical strains[J]. Hepatology, 2017, 66(6): 1826-1841. DOI: 10.1002/hep.29574. |
| [13] |
YURDAYD 1N C, IDILMAN R, BOZKAYA H, et al. Natural history and treatment of chronic delta hepatitis[J]. J Viral Hepat, 2010, 17(11): 749-756. DOI: 10.1111/j.1365-2893.2010.01353.x. |
| [14] |
FARCI P, NIRO GA. Clinical features of hepatitis D[J]. Semin Liver Dis, 2012, 32(3): 228-236. DOI: 10.1055/s-0032-1323628. |
| [15] |
BRICHLER S, LE GAL F, NERI-PINTO F, et al. Serological and molecular diagnosis of hepatitis delta virus infection: results of a French national quality control study[J]. J Clin Microbiol, 2014, 52(5): 1694-1697. DOI: 10.1128/JCM.03521-13. |
| [16] |
RICCO G, POPA DC, CAVALLONE D, et al. Quantification of serum markers of hepatitis B (HBV) and Delta virus (HDV) infections in patients with chronic HDV infection[J]. J Viral Hepat, 2018, 25(8): 911-919. DOI: 10.1111/jvh.12895. |
| [17] |
WRANKE A, HEIDRICH B, ERNST S, et al. Anti-HDV IgM as a marker of disease activity in hepatitis delta[J]. PLoS One, 2014, 9(7): e101002. DOI: 10.1371/journal.pone.0101002. |
| [18] |
GOWANS EJ, MACNAUGHTON TB, MICKAN L, et al. Use of recombinant hepatitis delta antigen in diagnostic assays for HDV antibody[J]. J Virol Methods, 1990, 27(1): 69-78. DOI: 10.1016/0166-0934(90)90147-8. |
| [19] |
TSWANA SA, LAHER S. Detection of anti-delta antibodies among acute hepatitis B virus-infected patients[J]. J Med Virol, 1988, 25(4): 471-474. DOI: 10.1002/jmv.1890250410. |
| [20] |
BEZEAUD A, ROSENSWAJG M, GUILLIN MC. Evaluation of five hepatitis delta virus marker assays for detection of antigen and antibody[J]. J Clin Microbiol, 1989, 27(12): 2880. DOI: 10.1128/jcm.27.12.2880-.1989. |
| [21] |
SHATTOCK AG, MORRIS MC. Evaluation of commercial enzyme immunoassays for detection of hepatitis delta antigen and anti-hepatitis delta virus (HDV) and immunoglobulin M anti-HDV antibodies[J]. J Clin Microbiol, 1991, 29(9): 1873-1876. DOI: 10.1128/jcm.29.9.1873-1876.1991. |
| [22] |
KUO YB, CHAO M, LEE YH, et al. New enzyme-linked immunosorbent assay for detection of antibodies against hepatitis delta virus using a hepatitis delta antigen derived from a Taiwanese clone and comparison to the Abbott radioimmunoassay[J]. Clin Vaccine Immunol, 2012, 19(5): 817-819. DOI: 10.1128/CVI.05687-11. |
| [23] |
GOVINDARAJAN S, VALINLUCK B, LAKE-BAKKAR G. Evaluation of a commercial anti-delta EIA kit for detection of antibodies to hepatitis delta virus[J]. Am J Clin Pathol, 1991, 95(2): 240-241. DOI: 10.1093/ajcp/95.2.240. |
| [24] |
DE ORY F, MINGUITO T, BALFAGÓN P, et al. Comparison of chemiluminescent immunoassay and ELISA for measles IgG and IgM[J]. APMIS, 2015, 123(8): 648-651. DOI: 10.1111/apm.12413. |
| [25] |
LEMPP FA, ROGGENBACH I, NKONGOLO S, et al. A rapid point-of-care test for the serodiagnosis of hepatitis delta virus infection[J]. Viruses, 2021, 13(12): 2371. DOI: 10.3390/v13122371. |
| [26] |
ROGGENBACH I, CHI X, LEMPP FA, et al. HDV seroprevalence in HBsAg-positive patients in China occurs in hotspots and is not associated with HCV mono-infection[J]. Viruses, 2021, 13(9): 1799. DOI: 10.3390/v13091799. |
| [27] |
CHEN X, OIDOVSAMBUU O, LIU P, et al. A novel quantitative microarray antibody capture assay identifies an extremely high hepatitis delta virus prevalence among hepatitis B virus-infected mongolians[J]. Hepatology, 2017, 66(6): 1739-1749. DOI: 10.1002/hep.28957. |
| [28] |
LU XX, YI Y, SU QD, et al. Expression and purification of recombinant hepatitis delta virus (HDV) antigen for use in a diagnostic ELISA for HDV infection using the high-density fermentation strategy in Escherichia coli[J]. Biomed Environ Sci, 2016, 29(6): 417-423. DOI: 10.3967/bes2016.054. |
| [29] |
WANG W, LEMPP FA, SCHLUND F, et al. Assembly and infection efficacy of hepatitis B virus surface protein exchanges in 8 hepatitis D virus genotype isolates[J]. J Hepatol, 2021, 75(2): 311-323. DOI: 10.1016/j.jhep.2021.03.025. |
| [30] |
COLLER KE, BUTLER EK, LUK KC, et al. Development and performance of prototype serologic and molecular tests for hepatitis delta infection[J]. Sci Rep, 2018, 8(1): 2095. DOI: 10.1038/s41598-018-20455-5. |
| [31] |
CHOW SK, ATIENZA EE, COOK L, et al. Comparison of enzyme immunoassays for detection of antibodies to hepatitis D virus in serum[J]. Clin Vaccine Immunol, 2016, 23(8): 732-734. DOI: 10.1128/CVI.00028-16. |
| [32] |
LE GAL F, BRICHLER S, SAHLI R, et al. First international external quality assessment for hepatitis delta virus RNA quantification in plasma[J]. Hepatology, 2016, 64(5): 1483-1494. DOI: 10.1002/hep.28772. |
| [33] |
ZHANG ZF, ZHUANG H. Research progress on laboratory diagnosis of hepatitis D[J]. Chin J Viral Dis, 2021, 11(6): 407-413. DOI: 10.16505/j.2095-0136.2021.0052. |



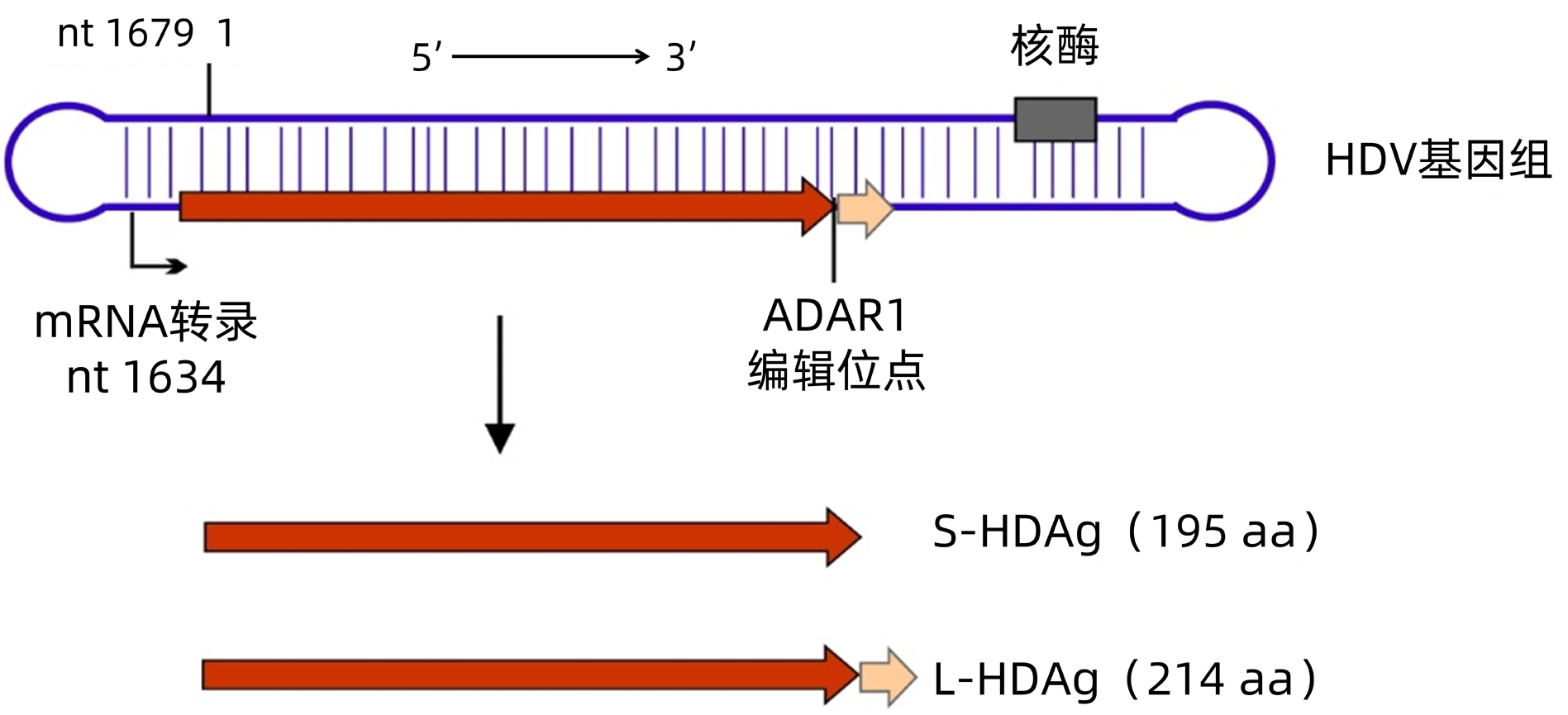




 DownLoad:
DownLoad: