| [1] |
URBAN S, NEUMANN-HAEFELIN C, LAMPERTICO P. Hepatitis D virus in 2021: virology, immunology and new treatment approaches for a difficult-to-treat disease[J]. Gut, 2021, 70(9): 1782-1794. DOI: 10.1136/gutjnl-2020-323888. |
| [2] |
MENTHA N, CLÉMENT S, NEGRO F, et al. A review on hepatitis D: From virology to new therapies[J]. J Adv Res, 2019, 17: 3-15. DOI: 10.1016/j.jare.2019.03.009. |
| [3] |
LEMPP FA, NI Y, URBAN S. Hepatitis delta virus: insights into a peculiar pathogen and novel treatment options[J]. Nat Rev Gastroenterol Hepatol, 2016, 13(10): 580-589. DOI: 10.1038/nrgastro.2016.126. |
| [4] |
WANG W, LEMPP FA, SCHLUND F, et al. Assembly and infection efficacy of hepatitis B virus surface protein exchanges in 8 hepatitis D virus genotype isolates[J]. J Hepatol, 2021, 75(2): 311-323. DOI: 10.1016/j.jhep.2021.03.025. |
| [5] |
STOCKDALE AJ, KREUELS B, HENRION M, et al. The global prevalence of hepatitis D virus infection: Systematic review and meta-analysis[J]. J Hepatol, 2020, 73(3): 523-532. DOI: 10.1016/j.jhep.2020.04.008. |
| [6] |
ZI J, GAO X, DU J, et al. Multiple regions drive hepatitis delta virus proliferation and are therapeutic targets[J]. Front Microbiol, 2022, 13: 838382. DOI: 10.3389/fmicb.2022.838382. |
| [7] |
GILLICH N, ZHANG Z, BINDER M, et al. Effect of variants in LGP2 on MDA5-mediated activation of interferon response and suppression of hepatitis D virus replication[J]. J Hepatol, 2023, 78(1): 78-89. DOI: 10.1016/j.jhep.2022.08.041. |
| [8] |
KOHSAR M, LANDAHL J, NEUMANN-HAEFELIN C, et al. Human hepatitis D virus-specific T cell epitopes[J]. JHEP Rep, 2021, 3(4): 100294. DOI: 10.1016/j.jhepr.2021.100294. |
| [9] |
|
| [10] |
TERRAULT NA, LOK A, MCMAHON BJ, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance[J]. Hepatology, 2018, 67(4): 1560-1599. DOI: 10.1002/hep.29800. |
| [11] |
CHEN LY, PANG XY, GOYAL H, et al. Hepatitis D: challenges in the estimation of true prevalence and laboratory diagnosis[J]. Gut Pathog, 2021, 13(1): 66. DOI: 10.1186/s13099-021-00462-0. |
| [12] |
LAZARUS JV, AL-RIFAI A, SANAI FM, et al. Hepatitis delta virus infection prevalence, diagnosis and treatment in the Middle East: A scoping review[J]. Liver Int, 2022. DOI: 10.1111/liv.15338.[Online ahead of print] |
| [13] |
VLACHOGIANNAKOS J, PAPATHEODORIDIS GV. New epidemiology of hepatitis delta[J]. Liver Int, 2020, 40 Suppl 1: 48-53. DOI: 10.1111/liv.14357. |
| [14] |
WRANKE A, HEIDRICH B, ERNST S, et al. Anti-HDV IgM as a marker of disease activity in hepatitis delta[J]. PLoS One, 2014, 9(7): e101002. DOI: 10.1371/journal.pone.0101002. |
| [15] |
CHEN X, OIDOVSAMBUU O, LIU P, et al. A novel quantitative microarray antibody capture assay identifies an extremely high hepatitis delta virus prevalence among hepatitis B virus-infected mongolians[J]. Hepatology, 2017, 66(6): 1739-1749. DOI: 10.1002/hep.28957. |
| [16] |
LEMPP FA, ROGGENBACH I, NKONGOLO S, et al. A rapid point-of-care test for the serodiagnosis of hepatitis delta virus infection[J]. Viruses, 2021, 13(12): 2371. DOI: 10.3390/v13122371. |
| [17] |
HOBLOS R, KEFALAKES H. Immunology of hepatitis D virus infection: General concepts and present evidence[J]. Liver Int, 2022. DOI: 10.1111/liv.15424.[Online ahead of print] |
| [18] |
DANDRI M, BERTOLETTI A, LVTGEHETMANN M. Innate immunity in hepatitis B and D virus infection: consequences for viral persistence, inflammation, and T cell recognition[J]. Semin Immunopathol, 2021, 43(4): 535-548. DOI: 10.1007/s00281-021-00864-x. |
| [19] |
YAO T, LV M, MA S, et al. Ubiquitinated hepatitis D antigen-loaded microvesicles induce a potent specific cellular immune response to inhibit HDV replication in vivo[J]. Microbiol Spectr, 2021, 9(3): e0102421. DOI: 10.1128/Spectrum.01024-21. |
| [20] |
XU L, ZHANG X, CAO Y, et al. Digital droplet PCR for detection and quantitation of hepatitis delta virus[J]. Clin Transl Gastroenterol, 2022, 13(7): e00509. DOI: 10.14309/ctg.0000000000000509. |
| [21] |
PFLVGER LS, NÖRZ D, VOLZ T, et al. Clinical establishment of a laboratory developed quantitative HDV PCR assay on the cobas6800 high-throughput system[J]. JHEP Rep, 2021, 3(6): 100356. DOI: 10.1016/j.jhepr.2021.100356. |
| [22] |
PALOM A, SOPENA S, RIVEIRO-BARCIELA M, et al. One-quarter of chronic hepatitis D patients reach HDV-RNA decline or undetectability during the natural course of the disease[J]. Aliment Pharmacol Ther, 2021, 54(4): 462-469. DOI: 10.1111/apt.16485. |
| [23] |
ZHANG Z, NI Y, LEMPP FA, et al. Hepatitis D virus-induced interferon response and administered interferons control cell division-mediated virus spread[J]. J Hepatol, 2022, 77(4): 957-966. DOI: 10.1016/j.jhep.2022.05.023. |
| [24] |
ROULOT D, BRICHLER S, LAYESE R, et al. Origin, HDV genotype and persistent viremia determine outcome and treatment response in patients with chronic hepatitis delta[J]. J Hepatol, 2020, 73(5): 1046-1062. DOI: 10.1016/j.jhep.2020.06.038. |
| [25] |
SCHWARZ C, CHROMY D, BANGERT C, et al. Immediate-type hypersensitivity reaction to bulevirtide and successful desensitization in a patient with HBV/HDV-associated compensated cirrhosis[J]. J Hepatol, 2022, 77(1): 254-255. DOI: 10.1016/j.jhep.2022.03.004. |
| [26] |
LIN YX, QIN YL, ZHANG JM. Clinical significance and methods of hepatitis D virus RNA detection[J]. J Clin Hepatol, 2022, 38(12): 2830-2835. DOI: 10.3969/j.issn.1001-5256.2022.12.028. |
| [27] |
KATSOULIDOU A, MANESIS E, ROKKA C, et al. Development and assessment of a novel real-time PCR assay for quantitation of hepatitis D virus RNA to study viral kinetics in chronic hepatitis D[J]. J Viral Hepat, 2013, 20(4): 256-262. DOI: 10.1111/jvh.12000. |
| [28] |
SCHOLTES C, ICARD V, AMIRI M, et al. Standardized one-step real-time reverse transcription-PCR assay for universal detection and quantification of hepatitis delta virus from clinical samples in the presence of a heterologous internal-control RNA[J]. J Clin Microbiol, 2012, 50(6): 2126-2128. DOI: 10.1128/JCM.06829-11. |
| [29] |
European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection[J]. J Hepatol, 2017, 67(2): 370-398. DOI: 10.1016/j.jhep.2017.03.021. |
| [30] |
SARIN SK, KUMAR M, LAU GK, et al. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update[J]. Hepatol Int, 2016, 10(1): 1-98. DOI: 10.1007/s12072-015-9675-4. |
| [31] |
RIZZETTO M, HAMID S, NEGRO F. The changing context of hepatitis D[J]. J Hepatol, 2021, 74(5): 1200-1211. DOI: 10.1016/j.jhep.2021.01.014. |
| [32] |
BÉGUELIN C, ATKINSON A, BOYD A, et al. Hepatitis delta infection among persons living with HIV in Europe[J]. Liver Int, 2023. DOI: 10.1111/liv.15519.[Online ahead of print] |



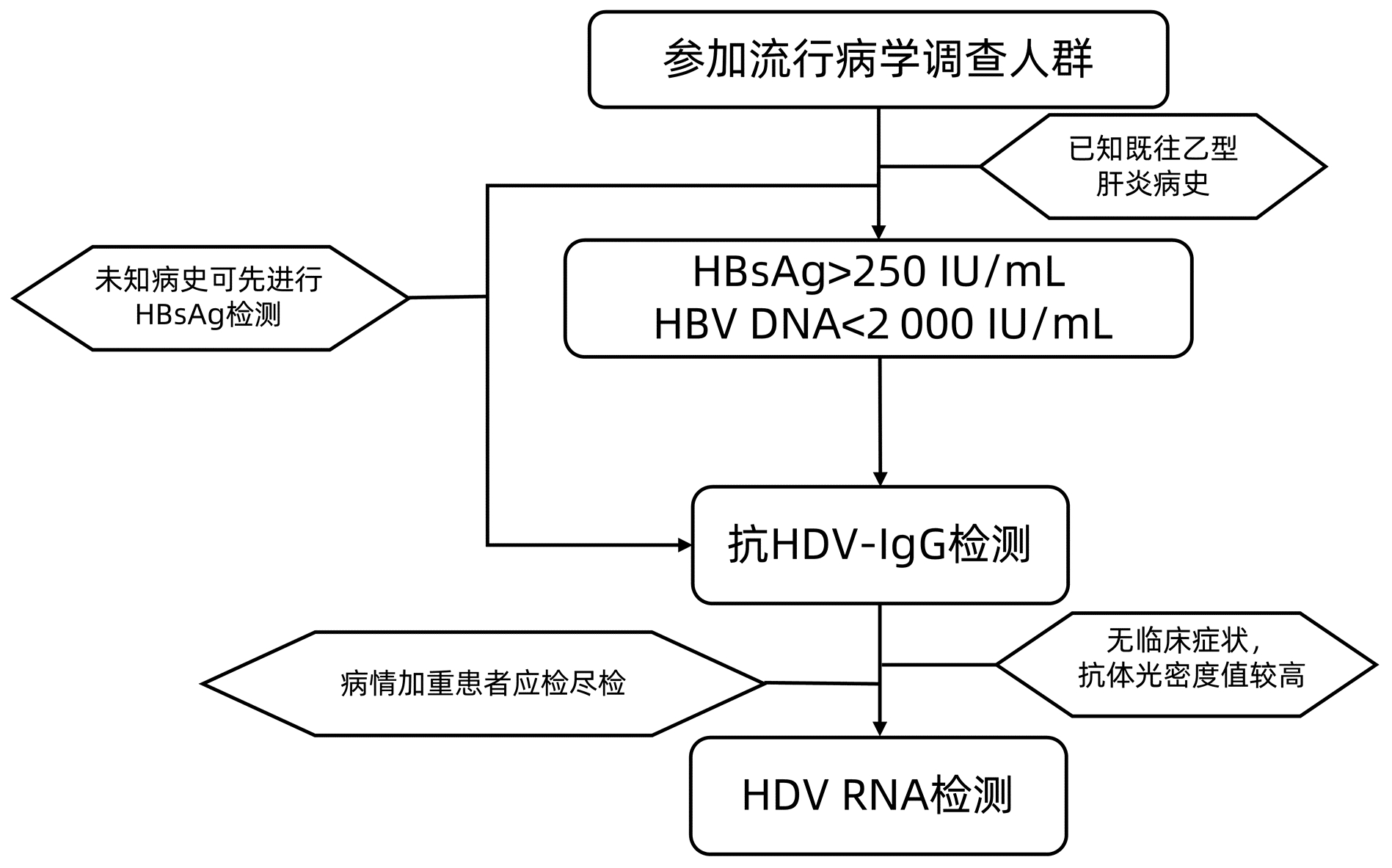




 DownLoad:
DownLoad: