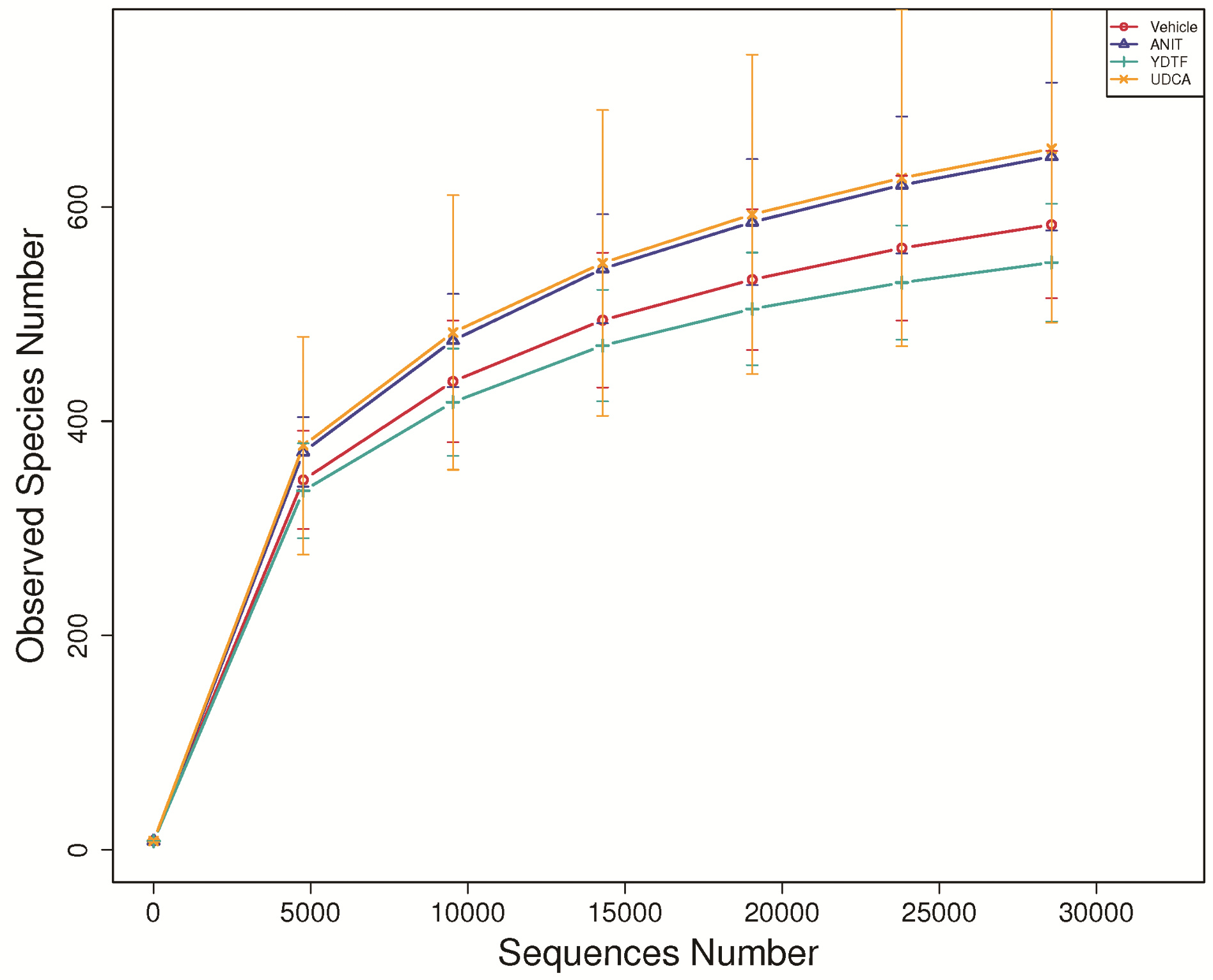
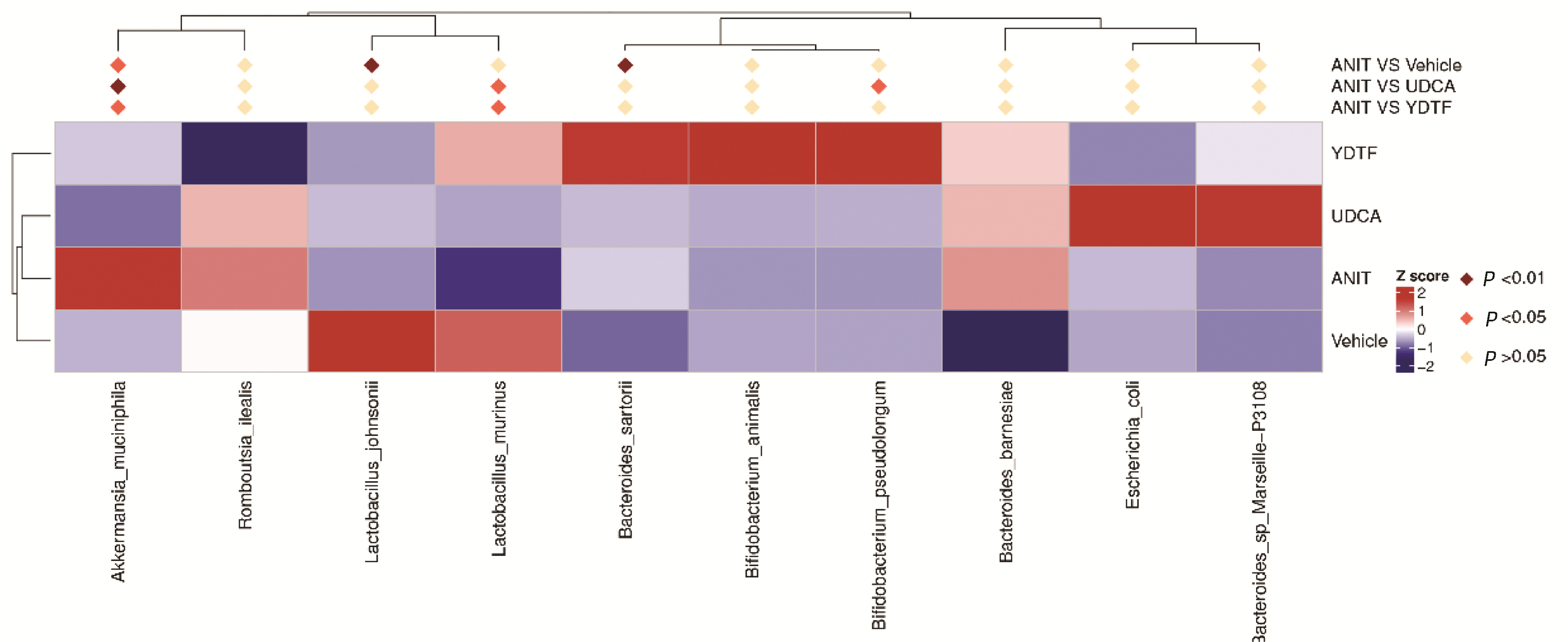
| [1] |
|
| [2] |
|
| [3] |
FANG KL, ZHENG XT, XU LP, et al. Experimental research progress of traditional chinese medicine in prevention and treatment of cholestatic liver disease[J]. Chin J Integr Tradit West Med Liver Dis, 2020, 30(4): 375-377. DOI: 10.3969/j.issn.1005-0264.2020.04.027. |
| [4] |
LARUSSO NF, TABIBIAN JH, O'HARA SP. Role of the intestinal microbiome in cholestatic liver disease[J]. Dig Dis, 2017, 35(3): 166-168. DOI: 10.1159/000450906. |
| [5] |
ZHOU R, FAN X, SCHNABL B. Role of the intestinal microbiome in liver fibrosis development and new treatment strategies[J]. Transl Res, 2019, 209: 22-38. DOI: 10.1016/j.trsl.2019.02.005. |
| [6] |
GUO C. Clinical and basic study on intestinal microecology of cholestasis[D]. Shijiazhuang: Hebei Medical University, 2020.
郭城. 胆汁淤积症肠道微生态学的临床与基础研究[D]. 石家庄: 河北医科大学, 2020.
|
| [7] |
HU Y, YAO Y, LIU J, et al. Pei Xueyi's experience in treating infantile hepatitis syndrome[J]. Chin J Inf Tradit Chin Med, 2012, 19(2): 87. DOI: 10.3969/j.issn.1005-5304.2012.02.041. |
| [8] |
CHEN L, HU Y, YANG M, et al. Interventional effect of traditional Chinese medicine on infants with biliary atresia after operation and its long-term effect[J]. JETCM, 2016, 25(2): 353-356. DOI: 10.3969/j.issn.1004-745X.2016.02.060. |
| [9] |
HU Y, CHEN L, SHU J, et al. Clinical observation on 60 cases of infant cytomegalovirus hepatitis treated with traditional Chinese medicine[J]. Chin Pediatr Integr Tradit Wset Med, 2012, 4(1): 98-99. DOI: 10.3969/j.issn.1674-3865.2012.02.002. |
| [10] |
|
| [11] |
LUO YS, ZHENG XT, ZHANG HY, et al. The cholestatic fibrosis induced by α-naphthylisothiocyanate in mice and the inflammation pathway[J]. CJAP, 2020, 36(2): 152-157. DOI: 10.12047/j.cjap.5903.2020.034. |
| [12] |
|
| [13] |
LIAO L, SCHNEIDER KM, GALVEZ E, et al. Intestinal dysbiosis augments liver disease progression via NLRP3 in a murine model of primary sclerosing cholangitis[J]. Gut, 2019, 68(8): 1477-1492. DOI: 10.1136/gutjnl-2018-316670. |
| [14] |
SWANSON KV, DENG M, TING JP. The NLRP3 inflammasome: molecular activation and regulation to therapeutics[J]. Nat Rev Immunol, 2019, 19(8): 477-489. DOI: 10.1038/s41577-019-0165-0. |
| [15] |
|
| [16] |
ISAACS-TEN A, ECHEANDIA M, MORENO-GONZALEZ M, et al. Intestinal microbiome-macrophage crosstalk contributes to cholestatic liver disease by promoting intestinal permeability[J]. Hepatology, 2020, 72(6): 2090-2108. DOI: 10.1002/hep.31228. |
| [17] |
HAO H, CAO L, JIANG C, et al. Farnesoid X receptor regulation of the NLRP3 inflammasome underlies cholestasis-associated sepsis[J]. Cell Metab, 2017, 25(4): 856-867. e5. DOI: 10.1016/j.cmet.2017.03.007. |
| [18] |
HUANG F, ZHENG X, MA X, et al. Theabrownin from Pu-erh tea attenuates hypercholesterolemia via modulation of gut microbiota and bile acid metabolism[J]. Nat Commun, 2019, 10(1): 4971. DOI: 10.1038/s41467-019-12896-x. |
| [19] |
WAHLSTRÖM A, KOVATCHEVA-DATCHARY P, STÅHLMAN M, et al. Crosstalk between bile acids and gut microbiota and its impact on farnesoid X receptor signalling[J]. Dig Dis, 2017, 35(3): 246-250. DOI: 10.1159/000450982. |
| [20] |
LI SL. Effect of FXR on LPS-induced macrophage inflammatory response and intestinal barrier injury in mice[D]. Chinese People's Liberation Army (PLA) Medical School, 2019.
李淑玲. FXR对LPS诱导的巨噬细胞炎症反应及小鼠肠道屏障损伤的作用研究[D]. 中国人民解放军医学院, 2019.
|
| [21] |
VERBEKE L, FARRE R, VERBINNEN B, et al. The FXR agonist obeticholic acid prevents gut barrier dysfunction and bacterial translocation in cholestatic rats[J]. Am J Pathol, 2015, 185(2): 409-419. DOI: 10.1016/j.ajpath.2014.10.009. |
| [22] |
ZHOU JL, WANG ZX, ZHOU SM, et al. Composition and functional change of intestinal microbiota in infantile cholestasis[J]. J Clin Hepatol, 2021, 37(1): 126-130. DOI: 10.3969/j.issn.1001-5256.2021.01.025. |
| [23] |
PAN F, ZHANG L, LI M, et al. Predominant gut Lactobacillus murinus strain mediates anti-inflammaging effects in calorie-restricted mice[J]. Microbiome, 2018, 6(1): 54. DOI: 10.1186/s40168-018-0440-5. |
| [24] |
WANG H, HE S, XIN J, et al. Psychoactive effects of lactobacillus johnsonii against restraint stress-induced memory dysfunction in mice through modulating intestinal inflammation and permeability-a study based on the gut-brain axis hypothesis[J]. Front Pharmacol, 2021, 12: 662148. DOI: 10.3389/fphar.2021.662148. |
| [25] |
ZHANG H, LIU M, LIU X, et al. Bifidobacterium animalis ssp. lactis 420 mitigates autoimmune hepatitis through regulating intestinal barrier and liver immune cells[J]. Front Immunol, 2020, 11: 569104. DOI: 10.3389/fimmu.2020.569104. |
| [26] |
MANGIN I, DOSSOU-YOVO F, LÉVÊQUE C, et al. Oral administration of viable Bifidobacterium pseudolongum strain Patronus modified colonic microbiota and increased mucus layer thickness in rat[J]. FEMS Microbiol Ecol, 2018, 94(11): fiy177. DOI: 10.1093/femsec/fiy177. |
| [27] |
SEREGIN SS, GOLOVCHENKO N, SCHAF B, et al. NLRP6 protects Il10 -/- mice from colitis by limiting colonization of akkermansia muciniphila[J]. Cell Rep, 2017, 19(4): 733-745. DOI: 10.1016/j.celrep.2017.03.080. |








 DownLoad:
DownLoad: