| [1] |
SUNG H, FERLAY J, SIEGEL RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA Cancer J Clin, 2021, 71(3): 209-249. DOI: 10.3322/caac.21660. |
| [2] |
Committee of Liver Transplantation, Chinese College of Transplant Doctors, Chinese Medical Doctor Association; Section of Liver Transplantation, Chinese Society of Organ Transplantation, Chinese Medical Association. Chinese expert consensus on application of sirolimus in liver transplantation for hepatocellular carcinoma(2020 edition)[J]. J Clin Hepatol, 2020, 36(11): 2429-2434. DOI: 10.3969/j.issn.1001-5256.2020.11.007. |
| [3] |
EL-KHOUEIRY AB, SANGRO B, YAU T, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial[J]. Lancet, 2017, 389(10088): 2492-2502. DOI: 10.1016/S0140-6736(17)31046-2. |
| [4] |
ZENG L, SU J, QIU W, et al. Survival outcomes and safety of programmed cell death/programmed cell death ligand 1 inhibitors for unresectable hepatocellular carcinoma: result from phase Ⅲ trials[J]. Cancer Control, 2022, 29: 10732748221092924. DOI: 10.1177/10732748221092924. |
| [5] |
YAU T, KANG YK, KIM TY, et al. Efficacy and safety of nivolumab plus ipilimumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib: the checkmate 040 randomized clinical trial[J]. JAMA Oncol, 2020, 6(11): e204564. DOI: 10.1001/jamaoncol.2020.4564. |
| [6] |
TSUNG I, WORDEN FP, FONTANA RJ. A pilot study of checkpoint inhibitors in solid organ transplant recipients with metastatic cutaneous squamous cell carcinoma[J]. Oncologist, 2021, 26(2): 133-138. DOI: 10.1002/onco.13539. |
| [7] |
LEONETTI A, WEVER B, MAZZASCHI G, et al. Molecular basis and rationale for combining immune checkpoint inhibitors with chemotherapy in non-small cell lung cancer[J]. Drug Resist Updat, 2019, 46: 100644. DOI: 10.1016/j.drup.2019.100644. |
| [8] |
MURAKAMI N, MULVANEY P, DANESH M, et al. A multi-center study on safety and efficacy of immune checkpoint inhibitors in cancer patients with kidney transplant[J]. Kidney Int, 2021, 100(1): 196-205. DOI: 10.1016/j.kint.2020.12.015. |
| [9] |
DOROSHOW DB, BHALLA S, BEASLEY MB, et al. PD-L1 as a biomarker of response to immune-checkpoint inhibitors[J]. Nat Rev Clin Oncol, 2021, 18(6): 345-362. DOI: 10.1038/s41571-021-00473-5. |
| [10] |
SHARMA P, SIDDIQUI BA, ANANDHAN S, et al. The next decade of immune checkpoint therapy[J]. Cancer Discov, 2021, 11(4): 838-857. DOI: 10.1158/2159-8290.CD-20-1680. |
| [11] |
LI X, WANG Y, YANG H, et al. Liver and hepatocyte transplantation: What can pigs contribute?[J]. Front Immunol, 2021, 12: 802692. DOI: 10.3389/fimmu.2021.802692. |
| [12] |
HAN CZ, WEI Q, XU X. Transplant oncology creates a new era of liver transplantation for the treatment of liver cancer[J]. J Clin Hepatol, 2021, 37(2): 253-256. DOI: 10.3969/j.issn.1001-5256.2021.02.002. |
| [13] |
JI R, DOU KF, XU H. Prevention and management of recurrence and metastasis of hepatocellular carcinoma after liver transplantation[J]. J Clin Hepatol, 2014, 30(1): 7-10. DOI: 10.3969/j.issn.1001-5256.2014.01.003. |
| [14] |
JIANG C, JING S, ZHOU H, et al. Efficacy and prognostic factors of trans-arterial chemoembolization combined with stereotactic body radiation therapy for BCLC stage B hepatocellular carcinoma[J]. Front Oncol, 2021, 11: 640461. DOI: 10.3389/fonc.2021.640461. |
| [15] |
KUDO M. A new treatment option for intermediate-stage hepatocellular carcinoma with high tumor burden: initial lenvatinib therapy with subsequent selective TACE[J]. Liver Cancer, 2019, 8(5): 299-311. DOI: 10.1159/000502905. |
| [16] |
LAWAL G, XIAO Y, RAHNEMAI-AZAR AA, et al. The immunology of hepatocellular carcinoma[J]. Vaccines (Basel), 2021, 9(10): 1184. DOI: 10.3390/vaccines9101184. |
| [17] |
SCHWACHA-EIPPER B, MINCIUNA I, BANZ V, et al. Immunotherapy as a downstaging therapy for liver transplantation[J]. Hepatology, 2020, 72(4): 1488-1490. DOI: 10.1002/hep.31234. |
| [18] |
YE Q, LING S, ZHENG S, et al. Liquid biopsy in hepatocellular carcinoma: circulating tumor cells and circulating tumor DNA[J]. Mol Cancer, 2019, 18(1): 114. DOI: 10.1186/s12943-019-1043-x. |
| [19] |
PELIZZARO F, GAMBATO M, GRINGERI E, et al Management of hepatocellular carcinoma recurrence after liver transplantation[J]. Cancers (Basel), 2021, 13(19): 4882. DOI: 10.3390/cancers13194882. |
| [20] |
OWOYEMI I, VAUGHAN LE, COSTELLO CM, et al. Clinical outcomes of solid organ transplant recipients with metastatic cancers who are treated with immune checkpoint inhibitors: A single-center analysis[J]. Cancer, 2020, 126(21): 4780-4787. DOI: 10.1002/cncr.33134. |
| [21] |
AMJAD W, KOTIAH S, GUPTA A, et al. Successful treatment of disseminated hepatocellular carcinoma after liver transplantation with nivolumab[J]. J Clin Exp Hepatol, 2020, 10(2): 185-187. DOI: 10.1016/j.jceh.2019.11.009. |
| [22] |
ZHUANG L, MOU HB, YU LF, et al. Immune checkpoint inhibitor for hepatocellular carcinoma recurrence after liver transplantation[J]. Hepatobiliary Pancreat Dis Int, 2020, 19(1): 91-93. DOI: 10.1016/j.hbpd.2019.09.011. |
| [23] |
FINN RS, RYOO BY, MERLE P, et al. Pembrolizumab as second-line therapy in patients with advanced hepatocellular carcinoma in KEYNOTE-240: A randomized, double-blind, phase iii trial[J]. J Clin Oncol, 2020, 38(3): 193-202. DOI: 10.1200/JCO.19.01307. |
| [24] |
YAU T, PARK JW, FINN RS, et al. Nivolumab versus sorafenib in advanced hepatocellular carcinoma (CheckMate 459): a randomised, multicentre, open-label, phase 3 trial[J]. Lancet Oncol, 2022, 23(1): 77-90. DOI: 10.1016/S1470-2045(21)00604-5. |
| [25] |
DELYON J, ZUBER J, DORENT R, et al. Immune checkpoint inhibitors in transplantation-a case series and comprehensive review of current knowledge[J]. Transplantation, 2021, 105(1): 67-78. DOI: 10.1097/TP.0000000000003292. |
| [26] |
SHI GM, WANG J, HUANG XW, et al. Graft programmed death ligand 1 expression as a marker for transplant rejection following anti-programmed death 1 immunotherapy for recurrent liver tumors[J]. Liver Transpl, 2021, 27(3): 444-449. DOI: 10.1002/lt.25887. |
| [27] |
NORDNESS MF, HAMEL S, GODFREY CM, et al. Fatal hepatic necrosis after nivolumab as a bridge to liver transplant for HCC: Are checkpoint inhibitors safe for the pretransplant patient?[J]. Am J Transplant, 2020, 20(3): 879-883. DOI: 10.1111/ajt.15617. |
| [28] |
CHEN GH, WANG GB, HUANG F, et al. Pretransplant use of toripalimab for hepatocellular carcinoma resulting in fatal acute hepatic necrosis in the immediate postoperative period[J]. Transpl Immunol, 2021, 66: 101386. DOI: 10.1016/j.trim.2021.101386. |
| [29] |
BRAHMER JR, DRAKE CG, WOLLNER I, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates[J]. J Clin Oncol, 2010, 28(19): 3167-3175. DOI: 10.1200/JCO.2009.26.7609. |
| [30] |
GAO Q, ANWAR IJ, ABRAHAM N, et al. Liver transplantation for hepatocellular carcinoma after downstaging or bridging therapy with immune checkpoint inhibitors[J]. Cancers (Basel), 2021, 13(24): 6307. DOI: 10.3390/cancers13246307. |
| [31] |
MORITA M, FUJINO M, JIANG G, et al. PD-1/B7-H1 interaction contribute to the spontaneous acceptance of mouse liver allograft[J]. Am J Transplant, 2010, 10(1): 40-46. DOI: 10.1111/j.1600-6143.2009.02859.x. |
| [32] |
LIU ZB, WU JS, LIN DD, et al. Safety of PD-1 inhibitor in preoperative treatment of liver transplantation for liver cancer[J]. Ogran Transplant, 2021, 12(4): 445-449. DOI: 10.3969/j.issn.1674-7445.2021.04.011. |
| [33] |
ClinicalTrials. gov. Combination camrelizumab (SHR-1210) and apatinib for downstaging/bridging of HCC before liver transplant[EB/OL]. (2019-07-29)[2022-07-28]. https://clinicaltrials.gov/ct2/show/NCT04035876.
|
| [34] |
ClinicalTrials. gov. Pembrolizumab and LENvatinib in participants with hepatocellular carcinoma (HCC) before liver transplant (PLENTY202001)[EB/OL]. (2020-06-11)[2022-07-28]. https://clinicaltrials.gov/ct2/show/NCT04425226.
|
| [35] |
ClinicalTrials. gov. Atezolizumab and bevacizumab pre-liver transplantation for patients with hepatocellular carcinoma beyond milan criteria[EB/OL]. (2022-01-11)[2022-07-28]. https://clinicaltrials.gov/ct2/show/NCT05185505.
|
| [36] |
ClinicalTrials. gov. Durvalumab (MEDI4736) and tremelimumab for hepatocellular carcinoma in patients listed for a liver transplant[EB/OL]. (2021-08-30)[2022-07-28]. https://clinicaltrials.gov/ct2/show/NCT05027425.
|
| [37] |
ClinicalTrials. gov. Durvalumab and lenvatinib in participants with locally advanced and metastatic hepatocellular carcinoma (Dulect2020-1) (Dulect2020-1)[EB/OL]. (2020-06-23)[2022-07-28]. https://clinicaltrials.gov/ct2/show/NCT04443322.
|
| [38] |
ClinicalTrials. gov. Safety and efficacy of camrelizumab (Anti-PD-1 Antibody) in recurrent HCC after liver transplantation[EB/OL]. (2020-09-25)[2022-07-28]. https://clinicaltrials.gov/ct2/show/NCT04564313.
|
| [39] |
ClinicalTrials. gov. IMRT plus PD-1 blockade and lenvatinib for HCC with PVTT (Vp3) before liver transplantation (iPLENTY-pvtt)[EB/OL]. (2022-04-21)[2022-07-28]. https://clinicaltrials.gov/ct2/show/NCT05339581.
|
| [40] |
ClinicalTrials. gov. Sequential PD-1/PD-L1 inhibitor and LENvatinib in TLCT and refractory hepatoblastoma after chemotherapy (sPLENTY-pc)[EB/OL]. (2022-04-11)[2022-07-28]. https://clinicaltrials.gov/ct2/show/NCT05322187.
|



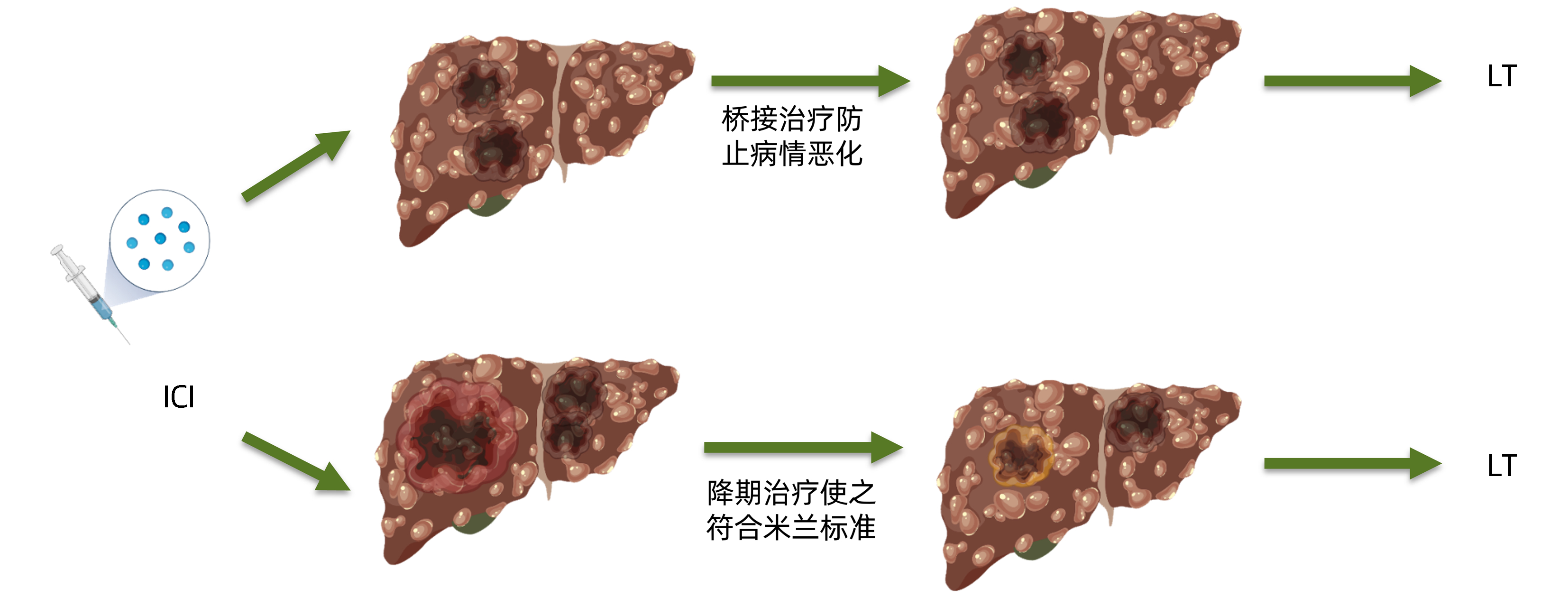




 DownLoad:
DownLoad: