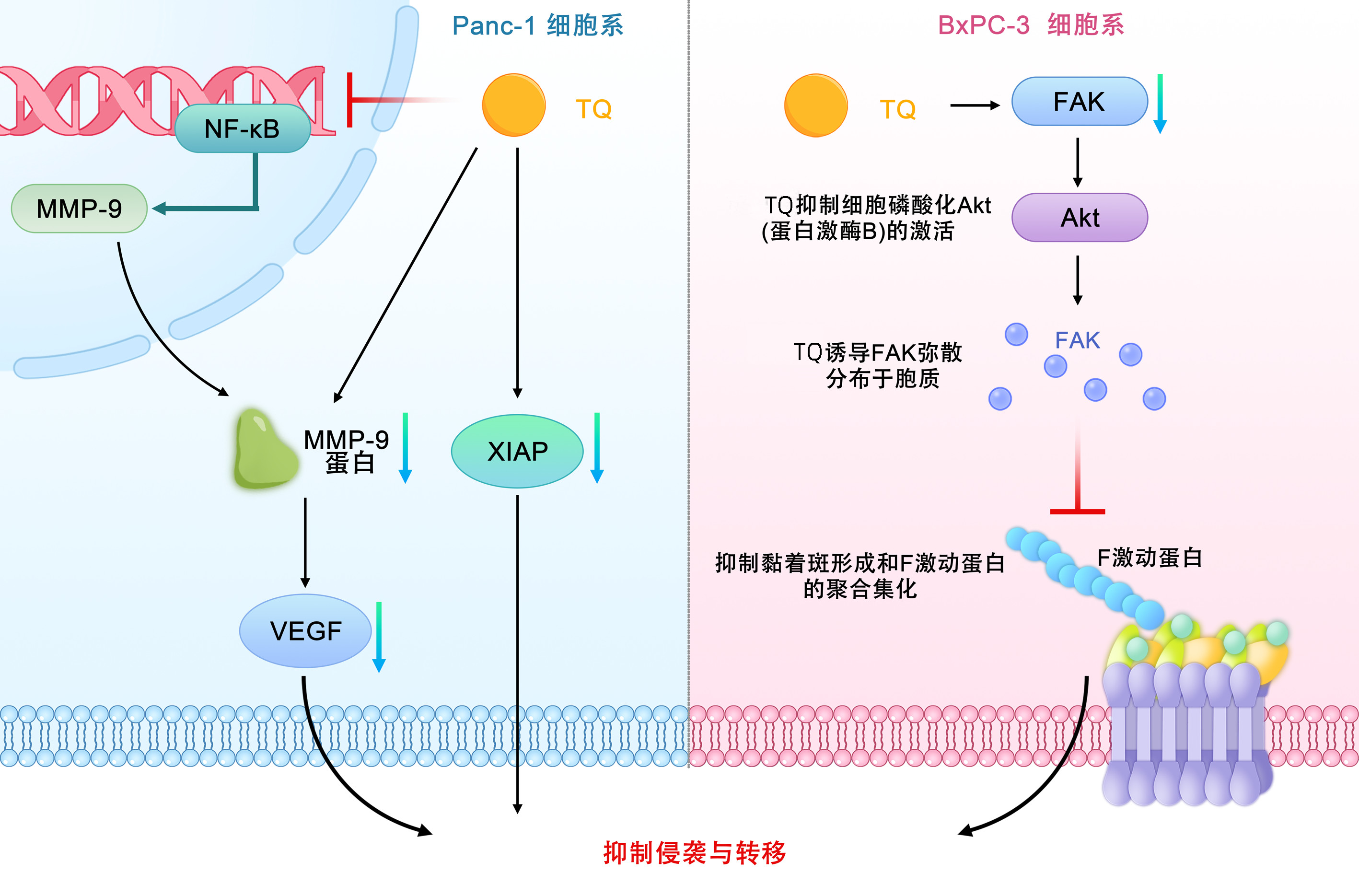
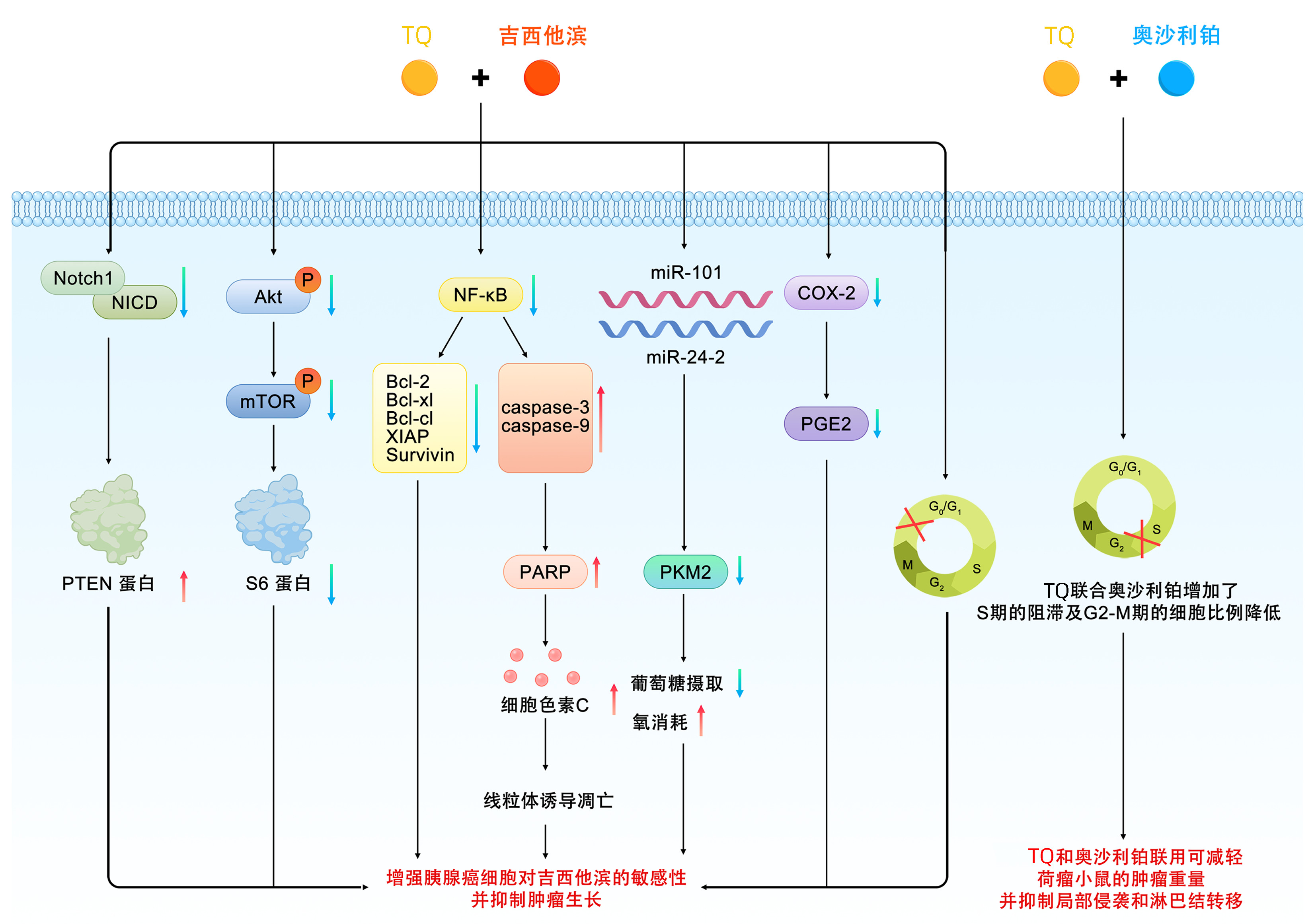
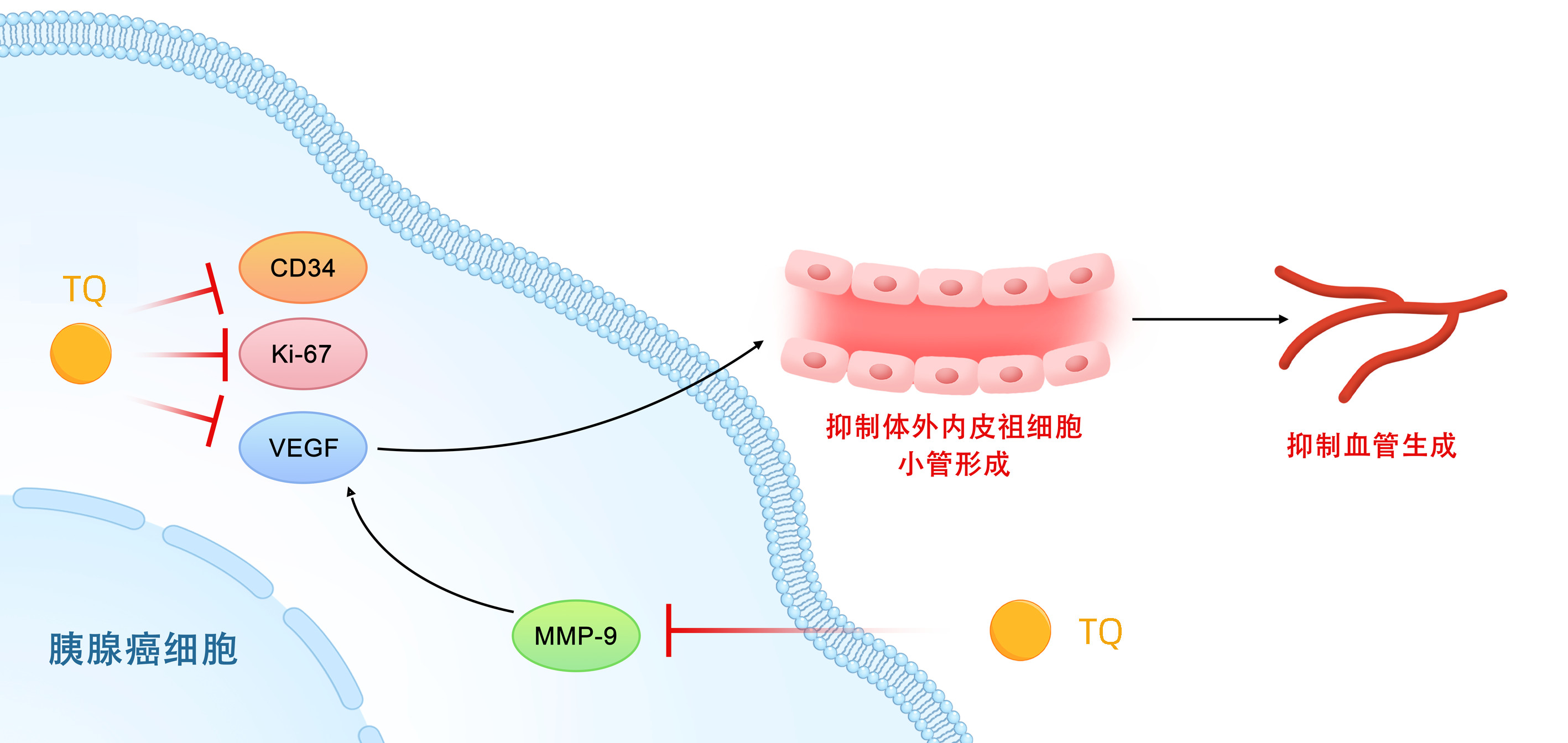
| [1] |
BRAY F, FERLAY J, SOERJOMATARAM I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA Cancer J Clin, 2018, 68(6): 394-424. DOI: 10.3322/caac.21492. |
| [2] |
SIEGEL RL, MILLER KD, JEMAL A. Cancer statistics, 2020[J]. CA Cancer J Clin, 2020, 70(1): 7-30. DOI: 10.3322/caac.21590. |
| [3] |
SUN D, CAO M, LI H, et al. Cancer burden and trends in China: A review and comparison with Japan and South Korea[J]. Chin J Cancer Res, 2020, 32(2): 129-139. DOI: 10.21147/j.issn.1000-9604.2020.02.01. |
| [4] |
Pancreatic Disease Collaborative Group, Chinese Society of Digestive Endoscopy. Chinese consensus on the early screening and surveillance for pancreatic cancer in high-risk individuals (2021, Nanjing)[J]. J Clin Hepatol, 2022, 38(5): 1016-1022. DOI: 10.3969/j.issn.1001-5256.2022.05.008. |
| [5] |
SHAFODINO FS, LUSILAO JM, MWAPAGHA LM. Phytochemical characterization and antimicrobial activity of Nigella sativa seeds[J]. PLoS One, 2022, 17(8): e0272457. DOI: 10.1371/journal.pone.0272457. |
| [6] |
LIU Y, HUANG L, KIM MY, et al. The role of thymoquinone in inflammatory response in chronic diseases[J]. Int J Mol Sci, 2022, 23(18): 10246. DOI: 10.3390/ijms231810246. |
| [7] |
ALAM M, HASAN GM, ANSAIR MM, et al. Therapeutic implications and clinical manifestations of thymoquinone. Phytochemistry[J]. 2022, 200: 113213. DOI: 10.1016/j.phytochem.2022.113213.
|
| [8] |
JAIN A, POOLADANDA V, BULBAKE U, et al. Liposphere mediated topical delivery of thymoquinone in the treatment of psoriasis[J]. Nanomedicine, 2017, 13(7): 2251-2262. DOI: 10.1016/j.nano.2017.06.009. |
| [9] |
PANG J, SHEN N, YAN F, et al. Thymoquinone exerts potent growth-suppressive activity on leukemia through DNA hypermethylation reversal in leukemia cells[J]. Oncotarget, 2017, 8(21): 34453-34467. DOI: 10.18632/oncotarget.16431. |
| [10] |
ALMAJALI B, AL-JAMAL H, TAIB W, et al. Thymoquinone, as a novel therapeutic candidate of cancers[J]. Pharmaceuticals (Basel), 2021, 14(4): 369. DOI: 10.3390/ph14040369. |
| [11] |
KANTER M. Thymoquinone attenuates lung injury induced by chronic toluene exposure in rats[J]. Toxicol Ind Health, 2011, 27(5): 387-395. DOI: 10.1177/0748233710387630. |
| [12] |
TAN M, NORWOOD A, MAY M, et al. Effects of (-)epigallocatechin gallate and thymoquinone on proliferation of a PANC-1 cell line in culture[J]. Biomed Sci Instrum, 2006, 42: 363-371.
|
| [13] |
TORRES MP, PONNUSAMY MP, CHAKRABORTY S, et al. Effects of thymoquinone in the expression of mucin 4 in pancreatic cancer cells: implications for the development of novel cancer therapies[J]. Mol Cancer Ther, 2010, 9(5): 1419-1431. DOI: 10.1158/1535-7163.MCT-10-0075. |
| [14] |
NARAYANAN P, FARGHADANI R, NYAMATHULLA S, et al. Natural quinones induce ROS-mediated apoptosis and inhibit cell migration in PANC-1 human pancreatic cancer cell line[J]. J Biochem Mol Toxicol, 2022, 36(5): e23008. DOI: 10.1002/jbt.23008. |
| [15] |
RELLES D, CHIPITSYNA GI, GONG Q, et al. Thymoquinone promotes pancreatic cancer cell death and reduction of tumor size through combined inhibition of histone deacetylation and induction of histone acetylation[J]. Adv Prev Med, 2016, 2016: 1407840. DOI: 10.1155/2016/1407840. |
| [16] |
IMRAN M, RAUF A, KHAN IA, et al. Thymoquinone: A novel strategy to combat cancer: A review[J]. Biomed Pharmacother, 2018(106): 390-402. DOI: 10.1016/j.biopha.2018.06.159. |
| [17] |
MU GG, ZHANG LL, LI HY, et al. Thymoquinone pretreatment overcomes the insensitivity and potentiates the antitumor effect of gemcitabine through abrogation of Notch1, PI3K/Akt/mTOR regulated signaling pathways in pancreatic cancer[J]. Dig Dis Sci, 2015, 60(4): 1067-1080. DOI: 10.1007/s10620-014-3394-x. |
| [18] |
KARKI N, AGGARWAL S, LAINE RA, et al. Cytotoxicity of juglone and thymoquinone against pancreatic cancer cells[J]. Chem Biol Interact, 2020, 327: 109142. DOI: 10.1016/j.cbi.2020.109142. |
| [19] |
MAHMOUD YK, ABDELRAZEK H. Cancer: Thymoquinone antioxidant/pro-oxidant effect as potential anticancer remedy[J]. Biomed Pharmacother, 2019, 115: 108783. DOI: 10.1016/j.biopha.2019.108783. |
| [20] |
WU ZH, CHEN Z, SHEN Y, et al. Anti-metastasis effect of thymoquinone on human pancreatic cancer[J]. Acta Pharm Sin, 2011, 46(8): 910-914.
吴志豪, 陈兆, 沈跃, 等. 百里醌抑制体内外胰腺癌转移作用[J]. 药学学报, 2011, 46(8): 910-914.
|
| [21] |
WANG YM. Inhibitory effects of thymoquinone on human pancreatic carcinoma orthotopically implanted in nude mice[J]. Nat Med J China, 2011, 91(44): 3111-3114. DOI: 10.3760/cma.j.issn.0376-2491.2011.44.005. |
| [22] |
MU GG, YU HG, LI HY, et al. Thymoquinone inhibits migration and invasion of human pancreatic cancer BxPC-3 cells in vitro[J]. Chin J Gastroenterol, 2014, 19(11): 650-654. DOI: 10.3969/j.issn.1008-7125.2014.11.003. |
| [23] |
FURUKAWA T. Mechanisms of development and progression of pancreatic neoplasms[J]. Pathol Int, 2022, 72(11): 529-540. DOI: 10.1111/pin.13272. |
| [24] |
SPALLAROSSA A, TASSO B, RUSSO E, et al. The development of FAK inhibitors: A five-year update[J]. Int J Mol Sci, 2022, 23(12): 6381. DOI: 10.3390/ijms23126381. |
| [25] |
MU GG, YU HG, LI HY, et al. Thymoquinone potentiates antitumor activity of gemcitabine in pancreatic cancer BxPC-3 cells in vitro[J]. J Med Res, 2014, 43(9): 72-76.
慕刚刚, 于红刚, 李红艳, 等. 百里醌联合吉西他滨对胰腺癌BxPC-3细胞体外生长的影响[J]. 医学研究杂志, 2014, 43(9): 72-76.
|
| [26] |
BANERJEE S, KASEB AO, WANG Z, et al. Antitumor activity of gemcitabine and oxaliplatin is augmented by thymoquinone in pancreatic cancer[J]. Cancer Res, 2009, 69(13): 5575-5583. DOI: 10.1158/0008-5472.CAN-08-4235. |
| [27] |
PANDITA A, KUMAR B, MANVATI S, et al. Synergistic combination of gemcitabine and dietary molecule induces apoptosis in pancreatic cancer cells and down regulates PKM2 expression[J]. PLoS One, 2014, 9(9): e107154. DOI: 10.1371/journal.pone.0107154. |
| [28] |
PANDITA A, MANVATI S, SINGH SK, et al. Combined effect of microRNA, nutraceuticals and drug on pancreatic cancer cell lines[J]. Chem Biol Interact, 2015, 233: 56-64. DOI: 10.1016/j.cbi.2015.03.018. |
| [29] |
WU ZH, XU Y, WANG ZH, et al. Effects of thymoquinone combined with gemcitabine on growth and apoptosis of human pancreatic cancer cell line BxPC-3[J]. Modern Pract Med, 2014, 10(6): 1242-1243. DOI: 10.3969/j.issn.1671-0800.2014.10.028 |
| [30] |
CUI J, GUO Y, WU H, et al. Everolimus regulates the activity of gemcitabine-resistant pancreatic cancer cells by targeting the Warburg effect via PI3K/AKT/mTOR signaling[J]. Mol Med, 2021, 27(1): 38. DOI: 10.1186/s10020-021-00300-8. |
| [31] |
ANSARY J, GIAMPIERI F, FORBES-HERNANDEZ TY, et al. Nutritional value and preventive role of nigella sativa L. and its main component thymoquinone in cancer: An evidenced-based review of preclinical and clinical studies[J]. Molecules, 2021, 26(8): 2108. DOI: 10.3390/molecules26082108. |
| [32] |
TALBERT EE, LEWIS HL, FARREN MR, et al. Circulating monocyte chemoattractant protein-1 (MCP-1) is associated with cachexia in treatment-naïve pancreatic cancer patients[J]. J Cachexia Sarcopenia Muscle, 2018, 9(2): 358-368. DOI: 10.1002/jcsm.12251. |
| [33] |
LIU A, WANG W, CHEN Z, et al. Anti-angiogenic effect of thymoquinone on angiogenesis and proliferation of pancreatic cancer[J]. Chin J Pathophysiol, 2011, 27(12): 2281-2285. DOI: 10.3969/j.issn.1000-4718.2011.12.008. |
| [34] |
BANERJEE S, AZMI AS, PADHYE S, et al. Structure-activity studies on therapeutic potential of Thymoquinone analogs in pancreatic cancer[J]. Pharm Res, 2010, 27(6): 1146-1158. DOI: 10.1007/s11095-010-0145-3. |
| [35] |
RACHAMALLA HK, BHATTACHARYA S, AHMAD A, et al. Enriched pharmacokinetic behavior and antitumor efficacy of thymoquinone by liposomal delivery[J]. Nanomedicine (Lond), 2021, 16(8): 641-656. DOI: 10.2217/nnm-2020-0470. |
| [36] |
BALLOUT F, HABLI Z, RAHAL ON, et al. Thymoquinone-based nanotechnology for cancer therapy: promises and challenges[J]. Drug Discov Today, 2018, 23(5): 1089-1098. DOI: 10.1016/j.drudis.2018.01.043. |
| [37] |
RAMACHANDRAN S, THANGARAJAN S. Thymoquinone loaded solid lipid nanoparticles counteracts 3-Nitropropionic acid induced motor impairments and neuroinflammation in rat model of Huntington's disease[J]. Metab Brain Dis, 2018, 33(5): 1459-1470. DOI: 10.1007/s11011-018-0252-0. |
| [38] |
SINGH A, AHMAD I, AKHTER S, et al. Nanocarrier based formulation of Thymoquinone improves oral delivery: stability assessment, in vitro and in vivo studies[J]. Colloids Surf B Biointerfaces, 2013, 102: 822-832. DOI: 10.1016/j.colsurfb.2012.08.038. |
| [39] |
NG WK, SAIFUL YAZAN L, YAP LH, et al. Thymoquinone-loaded nanostructured lipid carrier exhibited cytotoxicity towards breast cancer cell lines (MDA-MB-231 and MCF-7) and cervical cancer cell lines (HeLa and SiHa)[J]. Biomed Res Int, 2015, 2015: 263131. DOI: 10.1155/2015/263131. |
| [40] |
ABDELWAHAB SI, SHEIKH BY, TAHA MM, et al. Thymoquinone-loaded nanostructured lipid carriers: preparation, gastroprotection, in vitro toxicity, and pharmacokinetic properties after extravascular administration[J]. Int J Nanomedicine, 2013, 8: 2163-2172. DOI: 10.2147/IJN.S44108. |
| [41] |
RATHORE C, UPADHYAY N, KAUNDAL R, et al. Enhanced oral bioavailability and hepatoprotective activity of thymoquinone in the form of phospholipidic nano-constructs[J]. Expert Opin Drug Deliv, 2020, 17(2): 237-253. DOI: 10.1080/17425247.2020.1716728. |
| [42] |
BHATTACHARYA S, AHIR M, PATRA P, et al. PEGylated-thymoquinone-nanoparticle mediated retardation of breast cancer cell migration by deregulation of cytoskeletal actin polymerization through miR-34a[J]. Biomaterials, 2015, 51: 91-107. DOI: 10.1016/j.biomaterials.2015.01.007. |
| [43] |
VERMA SK, RASTOGI S, JAVED K, et al. Nanothymoquinone, a novel hepatotargeted delivery system for treating CCl4 mediated hepatotoxicity in rats[J]. J Mater Chem B, 2013, 1(23): 2956-2966. DOI: 10.1039/c3tb20379d. |
| [44] |
ABU-DAHAB R, ODEH F, ISMAIL SI, et al. Preparation, characterization and antiproliferative activity of thymoquinone-beta-cyclodextrin self assembling nanoparticles[J]. Pharmazie, 2013, 68(12): 939-944.
|
| [45] |
ODEH F, ISMAIL SI, ABU-DAHAB R, et al. Thymoquinone in liposomes: a study of loading efficiency and biological activity towards breast cancer[J]. Drug Deliv, 2012, 19(8): 371-377. DOI: 10.3109/10717544.2012.727500. |
| [46] |
General Office of National Health Commission. Standard for diagnosis and treatment of pancreatic cancer (2022 edition)[J]. J Clin Hepatol, 2022, 38(5): 1006-1015. DOI: 10.3969/j.issn.1001-5256.2022.05.007. |
| [47] |
|
| [48] |
ZHANG TP, LIU YZ, REN B. Current status and challenges of total neoadjuvant therapy for pancreatic cancer[J]. Chin J Dig Surg, 2022, 21(4): 461-464. DOI: 10.3760/cma.j.cn115610-20220320-00141. |
| [49] |
BUTNARIU M, QUISPE C, HERRERA-BRAVO J, et al. The effects of thymoquinone on pancreatic cancer: Evidence from preclinical studies[J]. Biomed Pharmacother, 2022, 153: 113364. DOI: 10.1016/j.biopha.2022.113364. |



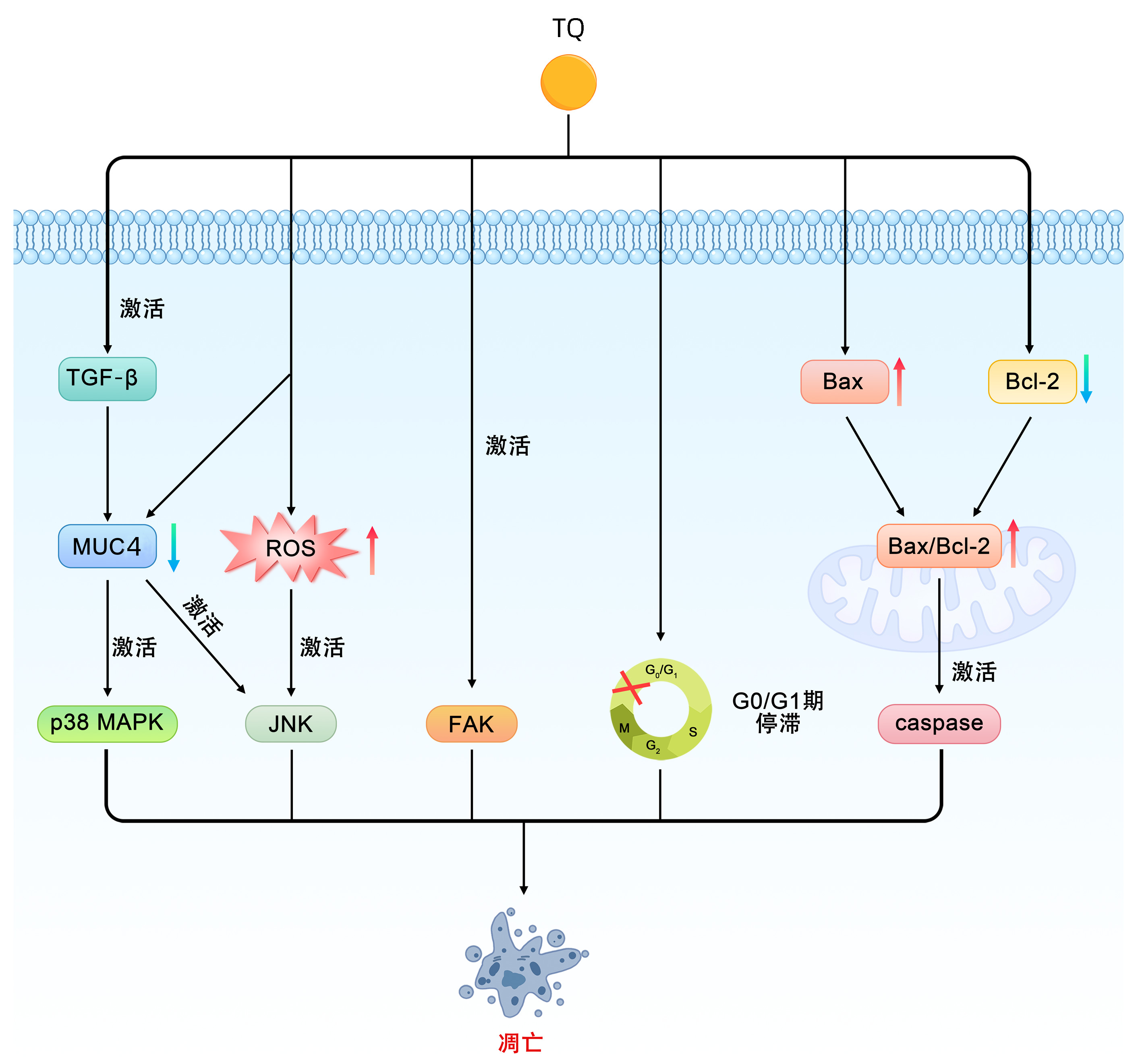




 DownLoad:
DownLoad: