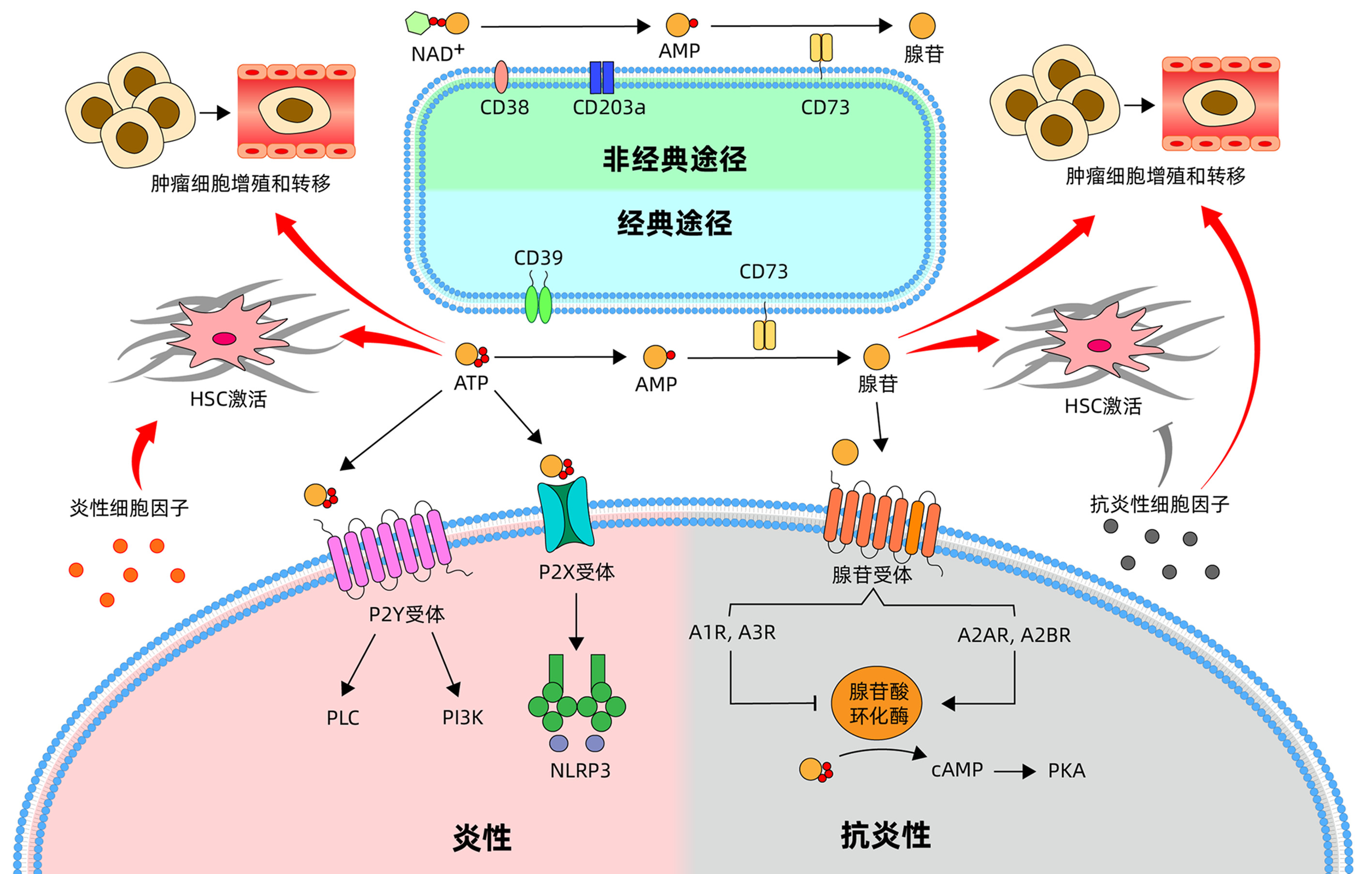
| [1] |
WANG P, JIA J, ZHANG D. Purinergic signalling in liver diseases: Pathological functions and therapeutic opportunities[J]. JHEP Rep, 2020, 2(6): 100165. DOI: 10.1016/j.jhepr.2020.100165. |
| [2] |
SHUAI Z, LEUNG MW, HE X, et al. Adaptive immunity in the liver[J]. Cell Mol Immunol, 2016, 13(3): 354-368. DOI: 10.1038/cmi.2016.4. |
| [3] |
LINDEN J, KOCH-NOLTE F, DAHL G. Purine release, metabolism, and signaling in the inflammatory response[J]. Annu Rev Immunol, 2019, 37: 325-347. DOI: 10.1146/annurev-immunol-051116-052406. |
| [4] |
DI VIRGILIO F, DAL BEN D, SARTI AC, et al. The P2X7 receptor in infection and inflammation[J]. Immunity, 2017, 47(1): 15-31. DOI: 10.1016/j.immuni.2017.06.020. |
| [5] |
MÜLLER T, FAY S, VIEIRA RP, et al. The purinergic receptor subtype P2Y2 mediates chemotaxis of neutrophils and fibroblasts in fibrotic lung disease[J]. Oncotarget, 2017, 8(22): 35962-35972. DOI: 10.18632/oncotarget.16414. |
| [6] |
ALBERTO AV, FARIA RX, DE MENEZES JR, et al. Role of P2 receptors as modulators of rat eosinophil recruitment in allergic inflammation[J]. PLoS One, 2016, 11(1): e0145392. DOI: 10.1371/journal.pone.0145392. |
| [7] |
BOREA PA, GESSI S, MERIGHI S, et al. Pharmacology of adenosine receptors: The state of the art[J]. Physiol Rev, 2018, 98(3): 1591-1625. DOI: 10.1152/physrev.00049.2017. |
| [8] |
KIRLEY TL, CRAWFORD PA, SMITH TM. The structure of the nucleoside triphosphate diphosphohydrolases (NTPDases) as revealed by mutagenic and computational modeling analyses[J]. Purinergic Signal, 2006, 2(2): 379-389. DOI: 10.1007/s11302-005-5301-6. |
| [9] |
ALLARD B, LONGHI MS, ROBSON SC, et al. The ectonucleotidases CD39 and CD73: Novel checkpoint inhibitor targets[J]. Immunol Rev, 2017, 276(1): 121-144. DOI: 10.1111/imr.12528. |
| [10] |
ANTONIOLI L, PACHER P, VIZI ES, et al. CD39 and CD73 in immunity and inflammation[J]. Trends Mol Med, 2013, 19(6): 355-367. DOI: 10.1016/j.molmed.2013.03.005. |
| [11] |
SYNNESTVEDT K, FURUTA GT, COMERFORD KM, et al. Ecto-5'-nucleotidase (CD73) regulation by hypoxia-inducible factor-1 mediates permeability changes in intestinal epithelia[J]. J Clin Invest, 2002, 110(7): 993-1002. DOI: 10.1172/JCI15337. |
| [12] |
CHALMIN F, MIGNOT G, BRUCHARD M, et al. Stat3 and Gfi-1 transcription factors control Th17 cell immunosuppressive activity via the regulation of ectonucleotidase expression[J]. Immunity, 2012, 36(3): 362-373. DOI: 10.1016/j.immuni.2011.12.019. |
| [13] |
ELTZSCHIG HK, KÖHLER D, ECKLE T, et al. Central role of Sp1-regulated CD39 in hypoxia/ischemia protection[J]. Blood, 2009, 113(1): 224-232. DOI: 10.1182/blood-2008-06-165746. |
| [14] |
PIEDRA-QUINTERO ZL, WILSON Z, NAVA P, et al. CD38: An immunomodulatory molecule in inflammation and autoimmunity[J]. Front Immunol, 2020, 11: 597959. DOI: 10.3389/fimmu.2020.597959. |
| [15] |
GOLDFINE ID, MADDUX BA, YOUNGREN JF, et al. The role of membrane glycoprotein plasma cell antigen 1/ectonucleotide pyrophosphatase phosphodiesterase 1 in the pathogenesis of insulin resistance and related abnormalities[J]. Endocr Rev, 2008, 29(1): 62-75. DOI: 10.1210/er.2007-0004. |
| [16] |
|
| [17] |
SNIDER NT, GRIGGS NW, SINGLA A, et al. CD73 (ecto-5'- nucleotidase) hepatocyte levels differ across mouse strains and contribute to mallory-denk body formation[J]. Hepatology, 2013, 58(5): 1790-1800. DOI: 10.1002/hep.26525. |
| [18] |
VELÁZQUEZ-MIRANDA E, DÍAZ-MUÑOZ M, VÁZQUEZ-CUEVAS FG. Purinergic signaling in hepatic disease[J]. Purinergic Signal, 2019, 15(4): 477-489. DOI: 10.1007/s11302-019-09680-3. |
| [19] |
ZOETEWIJ JP, VAN DE WATER B, DE BONT HJ, et al. The role of a purinergic P2z receptor in calcium-dependent cell killing of isolated rat hepatocytes by extracellular adenosine triphosphate[J]. Hepatology, 1996, 23(4): 858-865. DOI: 10.1002/hep.510230429. |
| [20] |
DIXON CJ, HALL JF, WEBB TE, et al. Regulation of rat hepatocyte function by P2Y receptors: focus on control of glycogen phosphorylase and cyclic AMP by 2-methylthioadenosine 5'-diphosphate[J]. J Pharmacol Exp Ther, 2004, 311(1): 334-341. DOI: 10.1124/jpet.104.067744. |
| [21] |
TACKETT BC, SUN H, MEI Y, et al. P2Y2 purinergic receptor activation is essential for efficient hepatocyte proliferation in response to partial hepatectomy[J]. Am J Physiol Gastrointest Liver Physiol, 2014, 307(11): G1073-G1087. DOI: 10.1152/ajpgi.00092.2014. |
| [22] |
FABRE AC, MALAVAL C, BEN ADDI A, et al. P2Y13 receptor is critical for reverse cholesterol transport[J]. Hepatology, 2010, 52(4): 1477-1483. DOI: 10.1002/hep.23897. |
| [23] |
GUINZBERG R, URIBE S, DÍAZ-CRUZ A, et al. In rat hepatocytes, different adenosine receptor subtypes use different secondary messengers to increase the rate of ureagenesis[J]. Life Sci, 2006, 79(4): 382-390. DOI: 10.1016/j.lfs.2006.01.021. |
| [24] |
BELDI G, WU Y, SUN X, et al. Regulated catalysis of extracellular nucleotides by vascular CD39/ENTPD1 is required for liver regeneration[J]. Gastroenterology, 2008, 135(5): 1751-1760. DOI: 10.1053/j.gastro.2008.07.025. |
| [25] |
ELTZSCHIG HK, THOMPSON LF, KARHAUSEN J, et al. Endogenous adenosine produced during hypoxia attenuates neutrophil accumulation: coordination by extracellular nucleotide metabolism[J]. Blood, 2004, 104(13): 3986-3992. DOI: 10.1182/blood-2004-06-2066. |
| [26] |
MCDONALD B, PITTMAN K, MENEZES GB, et al. Intravascular danger signals guide neutrophils to sites of sterile inflammation[J]. Science, 2010, 330(6002): 362-366. DOI: 10.1126/science.1195491. |
| [27] |
DRANOFF JA, OGAWA M, KRUGLOV EA, et al. Expression of P2Y nucleotide receptors and ectonucleotidases in quiescent and activated rat hepatic stellate cells[J]. Am J Physiol Gastrointest Liver Physiol, 2004, 287(2): G417-424. DOI: 10.1152/ajpgi.00294.2003. |
| [28] |
FAUSTHER M, LECKA J, SOLIMAN E, et al. Coexpression of ecto-5'-nucleotidase/CD73 with specific NTPDases differentially regulates adenosine formation in the rat liver[J]. Am J Physiol Gastrointest Liver Physiol, 2012, 302(4): G447-G459. DOI: 10.1152/ajpgi.00165.2011. |
| [29] |
SOHAIL MA, HASHMI AZ, HAKIM W, et al. Adenosine induces loss of actin stress fibers and inhibits contraction in hepatic stellate cells via Rho inhibition[J]. Hepatology, 2009, 49(1): 185-194. DOI: 10.1002/hep.22589. |
| [30] |
AHSAN MK, MEHAL WZ. Activation of adenosine receptor A2A increases HSC proliferation and inhibits death and senescence by down-regulation of p53 and Rb[J]. Front Pharmacol, 2014, 5: 69. DOI: 10.3389/fphar.2014.00069. |
| [31] |
CHE J, CHAN ES, CRONSTEIN BN. Adenosine A2A receptor occupancy stimulates collagen expression by hepatic stellate cells via pathways involving protein kinase A, Src, and extracellular signal-regulated kinases 1/2 signaling cascade or p38 mitogen-activated protein kinase signaling pathway[J]. Mol Pharmacol, 2007, 72(6): 1626-1636. DOI: 10.1124/mol.107.038760. |
| [32] |
CHAN ES, MONTESINOS MC, FERNANDEZ P, et al. Adenosine A(2A) receptors play a role in the pathogenesis of hepatic cirrhosis[J]. Br J Pharmacol, 2006, 148(8): 1144-1155. DOI: 10.1038/sj.bjp.0706812. |
| [33] |
SAVIO L, DE ANDRADE MELLO P, FIGLIUOLO VR, et al. CD39 limits P2X7 receptor inflammatory signaling and attenuates sepsis-induced liver injury[J]. J Hepatol, 2017, 67(4): 716-726. DOI: 10.1016/j.jhep.2017.05.021. |
| [34] |
BASU M, GUPTA P, DUTTA A, et al. Increased host ATP efflux and its conversion to extracellular adenosine is crucial for establishing Leishmania infection[J]. J Cell Sci, 2020, 133(7). DOI: 10.1242/jcs.239939. |
| [35] |
LU JC, ZHANG PF, HUANG XY, et al. Amplification of spatially isolated adenosine pathway by tumor-macrophage interaction induces anti-PD1 resistance in hepatocellular carcinoma[J]. J Hematol Oncol, 2021, 14(1): 200. DOI: 10.1186/s13045-021-01207-x. |
| [36] |
BIANCHI G, VUERICH M, PELLEGATTI P, et al. ATP/P2X7 axis modulates myeloid-derived suppressor cell functions in neuroblastoma microenvironment[J]. Cell Death Dis, 2014, 5(3): e1135. DOI: 10.1038/cddis.2014.109. |
| [37] |
VIJAYAN D, BARKAUSKAS D S, STANNARD K, et al. Selective activation of anti-CD73 mechanisms in control of primary tumors and metastases[J]. Oncoimmunology, 2017, 6(5): e1312044. DOI: 10.1080/2162402x.2017.1312044 |
| [38] |
CHATTERJEE D, TUFA DM, BAEHRE H, et al. Natural killer cells acquire CD73 expression upon exposure to mesenchymal stem cells[J]. Blood, 2014, 123(4): 594-595. DOI: 10.1182/blood-2013-09-524827. |
| [39] |
BELDI G, BANZ Y, KROEMER A, et al. Deletion of CD39 on natural killer cells attenuates hepatic ischemia/reperfusion injury in mice[J]. Hepatology, 2010, 51(5): 1702-1711. DOI: 10.1002/hep.23510. |
| [40] |
BASTID J, REGAIRAZ A, BONNEFOY N, et al. Inhibition of CD39 enzymatic function at the surface of tumor cells alleviates their immunosuppressive activity[J]. Cancer Immunol Res, 2015, 3(3): 254-265. DOI: 10.1158/2326-6066.CIR-14-0018. |
| [41] |
GRAUBARDT N, FAHRNER R, TROCHSLER M, et al. Promotion of liver regeneration by natural killer cells in a murine model is dependent on extracellular adenosine triphosphate phosphohydrolysis[J]. Hepatology, 2013, 57(5): 1969-1979. DOI: 10.1002/hep.26008. |
| [42] |
BEAVIS PA, DIVISEKERA U, PAGET C, et al. Blockade of A2A receptors potently suppresses the metastasis of CD73 + tumors[J]. Proc Natl Acad Sci U S A, 2013, 110(36): 14711-14716. DOI: 10.1073/pnas.1308209110. |
| [43] |
YOUNG A, NGIOW SF, GAO Y, et al. A2AR adenosine signaling suppresses natural killer cell maturation in the tumor microenvironment[J]. Cancer Res, 2018, 78(4): 1003-1016. DOI: 10.1158/0008-5472.CAN-17-2826. |
| [44] |
LOKSHIN A, RASKOVALOVA T, HUANG X, et al. Adenosine-mediated inhibition of the cytotoxic activity and cytokine production by activated natural killer cells[J]. Cancer Res, 2006, 66(15): 7758-7765. DOI: 10.1158/0008-5472.CAN-06-0478. |
| [45] |
RASKOVALOVA T, HUANG X, SITKOVSKY M, et al. Gs protein-coupled adenosine receptor signaling and lytic function of activated NK cells[J]. J Immunol, 2005, 175(7): 4383-4391. DOI: 10.4049/jimmunol.175.7.4383. |
| [46] |
NOBLE A, MEHTA H, LOVELL A, et al. IL-12 and IL-4 activate a CD39-dependent intrinsic peripheral tolerance mechanism in CD8(+) T cells[J]. Eur J Immunol, 2016, 46(6): 1438-1448. DOI: 10.1002/eji.201545939. |
| [47] |
SCHNEIDER E, WINZER R, RISSIEK A, et al. CD73-mediated adenosine production by CD8 T cell-derived extracellular vesicles constitutes an intrinsic mechanism of immune suppression[J]. Nat Commun, 2021, 12(1): 5911. DOI: 10.1038/s41467-021-26134-w. |
| [48] |
OHTA A, OHTA A, MADASU M, et al. A2A adenosine receptor may allow expansion of T cells lacking effector functions in extracellular adenosine-rich microenvironments[J]. J Immunol, 2009, 183(9): 5487-5493. DOI: 10.4049/jimmunol.0901247. |
| [49] |
SCHENK U, FRASCOLI M, PROIETTI M, et al. ATP inhibits the generation and function of regulatory T cells through the activation of purinergic P2X receptors[J]. Sci Signal, 2011, 4(162): ra12. DOI: 10.1126/scisignal.2001270. |
| [50] |
SAZE Z, SCHULER PJ, HONG CS, et al. Adenosine production by human B cells and B cell-mediated suppression of activated T cells[J]. Blood, 2013, 122(1): 9-18. DOI: 10.1182/blood-2013-02-482406. |
| [51] |
NASCIMENTO DC, VIACAVA PR, FERREIRA RG, et al. Sepsis expands a CD39 + plasmablast population that promotes immunosuppression via adenosine-mediated inhibition of macrophage antimicrobial activity[J]. Immunity, 2021, 54(9): 2024-2041. e8. DOI: 10.1016/j.immuni.2021.08.005. |
| [52] |
ZHANG F, LI R, YANG Y, et al. Specific decrease in B-cell-derived extracellular vesicles enhances post-chemotherapeutic CD8 + T cell responses[J]. Immunity, 2019, 50(3): 738-750. e7. DOI: 10.1016/j.immuni.2019.01.010. |
| [53] |
EYENGA P, REY B, EYENGA L, et al. Regulation of oxidative phosphorylation of liver mitochondria in sepsis[J]. Cells, 2022, 11(10): 1598. DOI: 10.3390/cells11101598. |
| [54] |
MORANDINI AC, SAVIO LE, COUTINHO-SILVA R. The role of P2X7 receptor in infectious inflammatory diseases and the influence of ectonucleotidases[J]. Biomed J, 2014, 37(4): 169-177. DOI: 10.4103/2319-4170.127803. |
| [55] |
AYATA CK, GANAL SC, HOCKENJOS B, et al. Purinergic P2Y 2 receptors promote neutrophil infiltration and hepatocyte death in mice with acute liver injury[J]. Gastroenterology, 2012, 143(6): 1620-1629. e4. DOI: 10.1053/j.gastro.2012.08.049. |
| [56] |
IDZKO M, FERRARI D, ELTZSCHIG HK. Nucleotide signalling during inflammation[J]. Nature, 2014, 509(7500): 310-317. DOI: 10.1038/nature13085. |
| [57] |
COHEN HB, BRIGGS KT, MARINO JP, et al. TLR stimulation initiates a CD39-based autoregulatory mechanism that limits macrophage inflammatory responses[J]. Blood, 2013, 122(11): 1935-1945. DOI: 10.1182/blood-2013-04-496216. |
| [58] |
NAGANUMA M, WIZNEROWICZ EB, LAPPAS CM, et al. Cutting edge: Critical role for A2A adenosine receptors in the T cell-mediated regulation of colitis[J]. J Immunol, 2006, 177(5): 2765-2769. DOI: 10.4049/jimmunol.177.5.2765. |
| [59] |
CSÓKA B, HIMER L, SELMECZY Z, et al. Adenosine A2A receptor activation inhibits T helper 1 and T helper 2 cell development and effector function[J]. FASEB J, 2008, 22(10): 3491-3499. DOI: 10.1096/fj.08-107458. |
| [60] |
ELTZSCHIG HK, IBLA JC, FURUTA GT, et al. Coordinated adenine nucleotide phosphohydrolysis and nucleoside signaling in posthypoxic endothelium: role of ectonucleotidases and adenosine A2B receptors[J]. J Exp Med, 2003, 198(5): 783-796. DOI: 10.1084/jem.20030891. |
| [61] |
TANG Y, JIANG L, ZHENG Y, et al. Expression of CD39 on FoxP3 + T regulatory cells correlates with progression of HBV infection[J]. BMC Immunol, 2012, 13: 17. DOI: 10.1186/1471-2172-13-17. |
| [62] |
RATHOD SB, DAS R, THANAPATI S, et al. Suppressive activity and altered conventional phenotype markers/mediators of regulatory T cells in patients with self-limiting hepatitis E[J]. J Viral Hepat, 2014, 21(2): 141-151. DOI: 10.1111/jvh.12125. |
| [63] |
WU X, WANG Y, WANG S, et al. Purinergic P2X7 receptor mediates acetaldehyde-induced hepatic stellate cells activation via PKC-dependent GSK3β pathway[J]. Int Immunopharmacol, 2017, 43: 164-171. DOI: 10.1016/j.intimp.2016.12.017. |
| [64] |
LE GUILCHER C, GARCIN I, DELLIS O, et al. The P2X4 purinergic receptor regulates hepatic myofibroblast activation during liver fibrogenesis[J]. J Hepatol, 2018, 69(3): 644-653. DOI: 10.1016/j.jhep.2018.05.020. |
| [65] |
PENG Z, FERNANDEZ P, WILDER T, et al. Ecto-5'-nucleotidase (CD73) -mediated extracellular adenosine production plays a critical role in hepatic fibrosis[J]. FASEB J, 2008, 22(7): 2263-2272. DOI: 10.1096/fj.07-100685. |
| [66] |
CHIANG DJ, ROYCHOWDHURY S, BUSH K, et al. Adenosine 2A receptor antagonist prevented and reversed liver fibrosis in a mouse model of ethanol-exacerbated liver fibrosis[J]. PLoS One, 2013, 8(7): e69114. DOI: 10.1371/journal.pone.0069114. |
| [67] |
CONNOLLY MK, BEDROSIAN AS, MALLEN-ST CLAIR J, et al. In liver fibrosis, dendritic cells govern hepatic inflammation in mice via TNF-alpha[J]. J Clin Invest, 2009, 119(11): 3213-3225. DOI: 10.1172/JCI37581. |
| [68] |
GIELING RG, WALLACE K, HAN YP. Interleukin-1 participates in the progression from liver injury to fibrosis[J]. Am J Physiol Gastrointest Liver Physiol, 2009, 296(6): G1324-G1331. DOI: 10.1152/ajpgi.90564.2008. |
| [69] |
LIU X, HU H, YIN JQ. Therapeutic strategies against TGF-beta signaling pathway in hepatic fibrosis[J]. Liver Int, 2006, 26(1): 8-22. DOI: 10.1111/j.1478-3231.2005.01192.x. |
| [70] |
CUI Q, WANG Z, JIANG D, et al. HGF inhibits TGF-β1-induced myofibroblast differentiation and ECM deposition via MMP-2 in Achilles tendon in rat[J]. Eur J Appl Physiol, 2011, 111(7): 1457-1463. DOI: 10.1007/s00421-010-1764-4. |
| [71] |
MAYNARD JP, LEE JS, SOHN BH, et al. P2X3 purinergic receptor overexpression is associated with poor recurrence-free survival in hepatocellular carcinoma patients[J]. Oncotarget, 2015, 6(38): 41162-41179. DOI: 10.18632/oncotarget.6240. |
| [72] |
KHALID M, BRISSON L, TARIQ M, et al. Carcinoma-specific expression of P2Y11 receptor and its contribution in ATP-induced purinergic signalling and cell migration in human hepatocellular carcinoma cells[J]. Oncotarget, 2017, 8(23): 37278-37290. DOI: 10.18632/oncotarget.16191. |
| [73] |
MA XL, SHEN MN, HU B, et al. CD73 promotes hepatocellular carcinoma progression and metastasis via activating PI3K/AKT signaling by inducing Rap1-mediated membrane localization of P110β and predicts poor prognosis[J]. J Hematol Oncol, 2019, 12(1): 37. DOI: 10.1186/s13045-019-0724-7. |



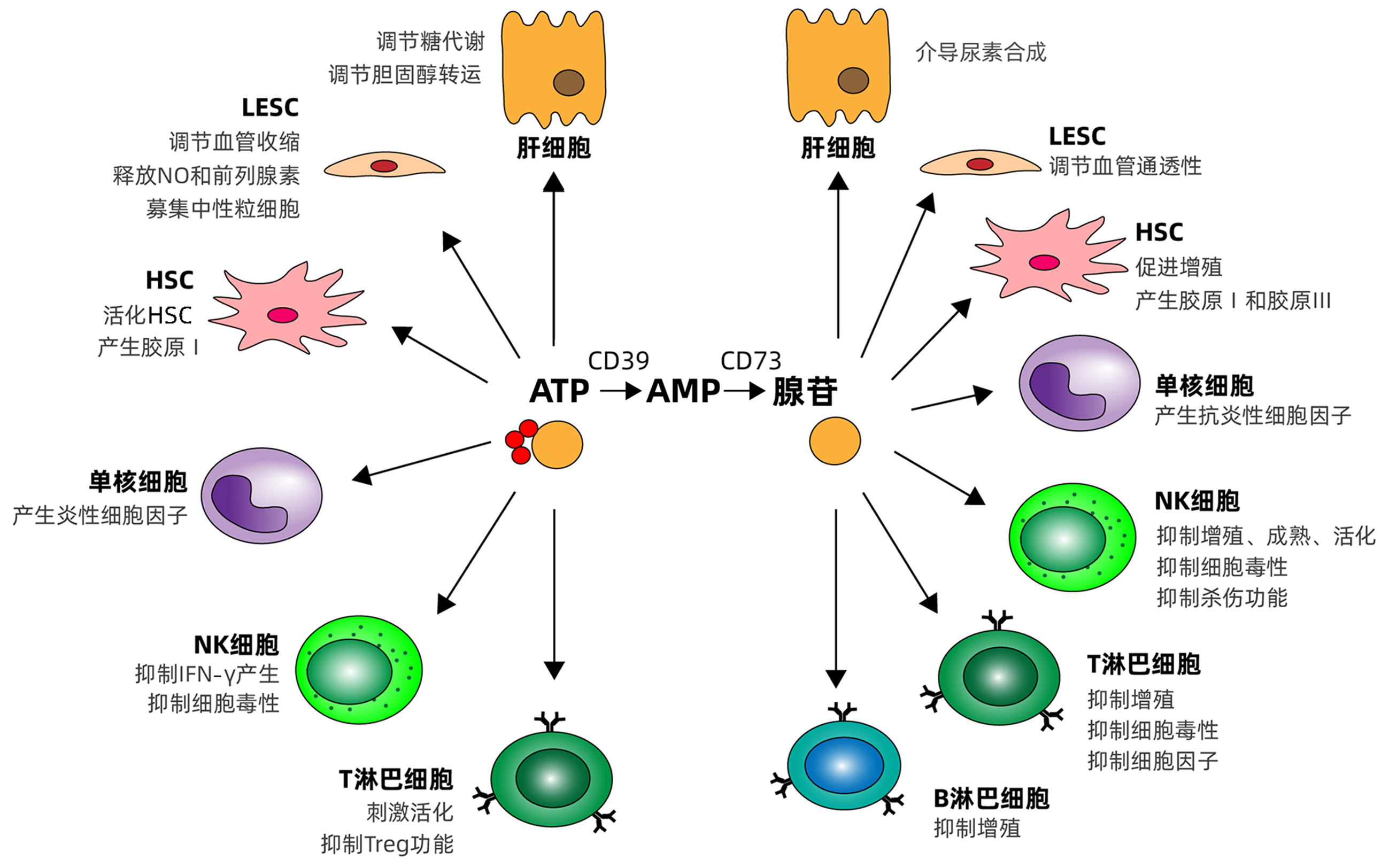




 DownLoad:
DownLoad: