非酒精性脂肪肝(Non-alcoholic fatty liver disease,NAFLD)是目前慢性肝病最常见的病因,影响到30%的普通人群和70-90%的高危人群[1,2]。该病疾病谱包括非酒精性肝脂肪变(Nonalcoholic HepaticSteatosis)、非酒精性脂肪性肝炎(Nonalcoholic Steatohepatitis,NASH)及NASH相关肝硬化和肝细胞癌。其中,非酒精性肝脂肪变一般被认为是NAFLD较为良性的阶段,发展为肝硬化的风险低且容易被逆转,但约25%的非酒精性肝脂肪变会进展为NASH[3],NASH发生后会导致肝纤维化进展、肝硬化,随后将导致肝功能衰竭和死亡[4]。有研究在超过8年的随访中发现,21-26%的NASH患者发展成肝硬化且由该疾病引起的肝脏相关死亡率是一般人群的10倍[5]。近年来,NASH也呈现出逐年升高的发病趋势[6]。其庞大的患病基数及逐年升高的发病率使得对该疾病的控制变得极其必要和紧迫。然而,迄今为止尚无针对NASH的有效药物批准上市[7],探索有效的抗NASH药物引发大家越来越来越多的关注,各国监管机构也针对该疾病颁布了相应的指南。
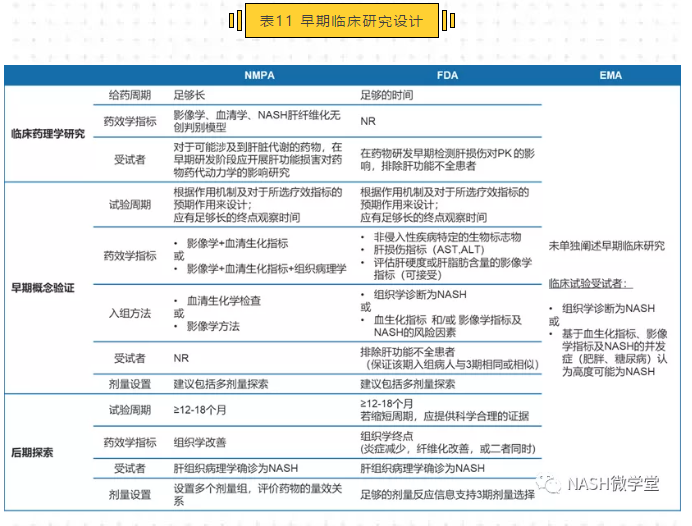
本文就中、美、欧三地监管部门制定的指导原则信息进行对比,以明确NASH疾病临床试验设计的法规要求。






参考文献
[1] Browning JD, SzczepaniakLS, Dobbins R, et al. Prevalence of hepatic steatosis in an urban population inthe United States: impact of ethnicity. Hepatology 2004;40:1387–95.
[2]Bellentani S,Bedogni G, Miglioli L, et al. The epidemiology of fatty liver. Eur JGastroenterol Hepatol 2004;16:1087–93.
[3]Williams CD, etal. Prevalence of nonalcoholic fatty liver disease and nonalcoholicsteatohepatitis among a largely middle-aged population utilizing ultrasound andliver biopsy: a prospective study. Gastroenterology. 2011;140(1):124-31.
[4]Williams CD,Stengel J, Asike MI, et al. Prevalence of non-alcoholic fatty liver disease andnonalcoholic steatohepatitis among a largely middle-aged population utilizingultrasound and liver biopsy: a prospective study. Gastroenterology 2011;140:124–31
[5] Matteoni CA,Younossi ZM, Gramlich T, et al. Nonalcoholic fatty liver disease: a spectrum ofclinical and pathological severity. Gastroenterology. 1999;116:1413–1419.
[6] Adam C. Sheka,; Oyedele Adeyi, et al. Nonalcoholic Steatohepatitis: A Review. The Journal ofthe American Medical Association. 2020;323(12):1175-1183.
[7] Musso G,Cassader M, Rosina F, et al. Impact of current treatments on liver disease,glucose metabolism and cardiovascular risk in non-alcoholic fatty liver disease(NAFLD): a systematic review and meta-analysis of randomised trials.Diabetologia 2012;55:885–904.
[8]Noncirrhotic Nonalcoholic Steatohepatitis With Liver Fibrosis: Developing Drugs for Treatment Guidance forIndustry –FDA
[9] Reflection paper on regulatory requirements forthe development of medicinal products for chronic non-infectious liver diseases(PBC,PSC, NASH).-EMA
[10]非酒精性脂肪性肝炎治疗药物临床试验指导原则(试行)201912