轻微肝性脑病患者经颈静脉肝内门体分流术后不同预后组肠道菌群的变化
DOI: 10.3969/j.issn.1001-5256.2021.02.016
Changes in gut microbiota after transjugular intrahepatic portosystemic shunt in cirrhotic patients with mild hepatic encephalopathy in different prognosis groups
-
摘要:
目的 对比分析肝硬化轻微肝性脑病(MHE)患者经颈静脉肝内门体分流术(TIPS)后不同预后组肠道菌群结构与术前的差异。 方法 选取2016年7月—2017年7月西京医院消化病院住院并接受TIPS治疗的28例MHE患者,分别于术前1~3 d和术后1个月收集粪便样本及采样时临床资料信息,根据术后预后不同分为3组:无HE组(n=8)、MHE组(n=12)和显性脑病(OHE)组(n=8)。对粪便样本采取16S rRNA高通量测序技术进行测序得到菌群相对丰度并在属水平使用SPSS和R语言对各组间菌群物种多样性,术后变化和变化差异展开分析。计数资料组间比较采用χ2检验,计量资料3组间比较采用Kruskal-Wallis H检验,采用Bonferroni法进行多个样本的多重比较,同组患者术前术后的比较采用Wilcoxo符号秩检验。微生物组Beta分析基于Bray Curtis距离矩阵进行主坐标分析(PCoA),使用Adonis法(PerMANOVA)对比组间差异。 结果 基于Bray Curtis距离矩阵的PCoA分析显示,仅MHE组术前和术后的beta多样性明显改变(F=2.71,P=0.049)。术后无HE组原生菌群小杆菌属、粪球菌属、瘤胃菌科某属、解黄酮菌属和狭义梭菌属丰度较术前明显升高(Z值分别为2.521、2.1、2.1、2.1和1.96,P值均<0.05);MHE组术后有害菌群颗粒链菌属(Z=2.521, P=0.012)、肠球菌属(Z=2.51, P=0.012)、链球菌属(Z=2.432, P=0.015)和罗氏菌属(Z=2.001, P=0.045)丰度较术前明显下降,但韦荣球菌属(Z=2.353, P=0.019)和巨球菌属(Z=1.955, P=0.05)丰度却明显上升; OHE组术后仅观察到韦荣球菌属丰度较术前升高(Z=2.38,P= 0.017)。3组间菌群变化量(术后丰度/术前丰度)比较差异有统计学意义[无HE组vs MHE组vs OHE组:2.00(1.11~91.61) vs 1.21(0.26~6.79) vs 0.09(0.01~0.92),χ2=6.249,P=0.043]。 结论 TIPS术后不同预后患者肠道菌群的变化有明显的差异,原生菌群丰度的升高可能对缓解HE病情有一定影响。 -
关键词:
- 肝硬化 /
- 肝性脑病 /
- 门体分流术, 经颈静脉肝内 /
- 胃肠道微生物组
Abstract:Objective To investigate the changes in gut microbiota after transjugular intrahepatic portosystemic shunt (TIPS) in cirrhotic patients with mild hepatic encephalopathy (MHE) in different prognosis groups. Methods A total of 28 MHE cirrhotic patients who were hospitalized and underwent TIPS in Xijing Hospital of Digestive Diseases from July 2016 to July 2017 were enrolled. Fecal samples and related clinical data were collected on days 1-3 before surgery and at 1 month after surgery. According to the prognosis after surgery, the patients were divided into none-hepatic encephalopathy (HE) group with 8 patients, MHE group with 12 patients, and overt hepatic encephalopathy (OHE) group with 8 patients. Fecal samples were analyzed by 16S rRNA sequencing to obtain the relative abundance of gut microbiota, and SPSS and R packages were used to analyze the biodiversity, postoperative changes, and differences in such changes of gut microbiota at the genus level between groups. The chi-square test was used for comparison of categorical data between groups; the Kruskal-Wallis H test was used for comparison of continuous data between three groups; the Bonferroni method was used for multiple comparisons of multiple samples; the Wilcoxon signed-rank test was used for comparison before and after surgery within each group. For microbiome beta-diversity analyses, a principal coordinate analysis (PCoA) was performed based on Bray-Curtis distance matrix, and the Adonis method (PerMANOVA) was used for comparison between groups. Results PCoA based on Bray-Curtis distance matrix showed that only the MHE group had a significant change in beta diversity after surgery (F=2.71, P=0.049). After surgery, the non-HE group had significant increases in the abundance of the native flora Dialister, Coprococcus, Ruminococcaceae_uncultured, Flavonifractor, and Clostridium_sensu_stricto_1 (Z=2.521, 2.1, 2.1, 2.1, and 1.96, all P < 0.05); the MHE group had significant reductions in the abundance of the harmful flora Granulicatella(Z=2.521, P=0.012), Enterococcus(Z=2.51, P=0.012), Streptococcus(Z=2.432, P=0.015), and Rothia(Z=2.001, P=0.045) and significant increases in the abundance of Veillonella(Z=2.353, P=0.019) and Megasphaera(Z=1.955, P=0.05); the OHE group only had a significant increase in the abundance of Veillonella after surgery (Z=2.38, P=0.017). There was a significant difference in the change in gut microbiota (postoperative abundance/preoperative abundance) between the non-HE group, the MHE group, and the OHE group [2.00 (1.11-91.61) vs 1.21 (0.26-6.79) vs 0.09 (0.01-0.92), χ2=6.249, P=0.043]. Conclusion There is a significant difference in the change in gut microbiota after TIPS between patients with different prognoses, and the increase in the abundance of native flora may have a certain influence on the remission of MHE. -
肝性脑病(HE)是继发于肝脏严重疾病所出现的以代谢紊乱为基础、轻重程度不同的神经精神异常综合征[1-2]。轻微肝性脑病(MHE)是HE症状最轻微的阶段,可降低患者生活质量,增加显性脑病(OHE)发生率和死亡风险。慢性肝硬化患者中MHE的患病率高达80%[3],住院肝硬化患者隐匿性HE的发病率为39.9%[4]。研究表明,肝硬化患者肠道菌群发生明显改变[5-7],肠道菌群失调随肝硬化严重程度加重而加重[8]。OHE患者和MHE患者的肠道菌群具有特征性变化,如有益菌/原生菌群减少,而有害菌/潜在致病菌增多等[9]。
经颈静脉肝内门体分流术(TIPS)是降低门静脉压力最有效的方式之一,可显著控制再出血率,减轻腹水程度,但是HE作为TIPS术后最主要的并发症,严重影响术后患者的生活和生存质量[1]。由于缺乏重视,TIPS术前很少对患者进行MHE检测,那么MHE患者TIPS术后预后如何,是否与肠道菌群的改变有关成为一个值得探讨的问题。因此,本文通过高通量测序方法,分析TIPS术后不同预后的患者其肠道菌群与术前的差异,探索从菌群角度预防和治疗MHE患者TIPS术后脑病的新策略。
1. 资料与方法
1.1 研究对象
选取2016年7月—2017年7月在本院接受TIPS治疗的MHE患者。MHE的诊断根据中华医学会肝病学分会制定的《肝硬化肝性脑病诊疗指南》[10],术前1~3 d及术后1个月对患者进行传统纸-笔神经心理学测试:HE心理学评分,包括数字连接试验(NCT) A、B, 数字符号试验(DST), 轨迹描绘试验, 系列打点试验5个子测试试验。若NCT-A、DST均阳性,或5个子试验中任何2项异常,即诊断为MHE。OHE诊断根据West-Haven标准及分级[11]。
1.2 纳入标准
(1) 经临床/实验室检查,影像学检查或肝活检确诊为肝硬化;(2)计划接受TIPS治疗以预防门静脉高压性静脉曲张再出血;(3)术前检测为MHE患者。
1.3 排除标准
(1) 肝脏及胃肠道恶性肿瘤;(2)术前腹泻、血便、便秘;(3)术前HE病史;(4)未控制的严重活动性感染(>2级)或败血症;(5)严重的心、肾、肺功能不全;(6)曾接受TIPS或手术分流史;(7)布加综合征;(8)入组前3个月胃肠道手术史;(9)近期计划肝移植手术;(10)怀孕或哺乳期受试者。
1.4 研究方法
分别于术前1~3 d和术后1个月收集粪便样本及采样时临床资料信息。采样前1 d嘱患者清淡饮食,使用无菌采样管收集新鲜粪便,液氮速冻后在超净工作台上预处理,取采样管中心部位样本3~5 g于样本管中,存于实验室-80 ℃冰箱。并于采样当天记录患者的临床资料信息。根据患者术后1个月内发生HE的类型进行分组。
1.5 粪便微生物DNA提取、鉴定以及16S rRNA高通量测序
便微生物DNA提取、鉴定以及16S rRNA测序由上海态昌基因科技有限公司完成。粪便样本DNA提取采用E.Z.N.A.®Stool DNA Kit (Omega Bio-tek, Inc., 美国),采用热循环PCR系统扩增细菌16S rRNA基因的V3-V4超变区,引物序列为338F (5′-ACTCCTACGGGAGGCAGCA-3′)和806R(5′-GGACTACHVGGGTWTCTAAT-3′),PCR反应采用EasyCycler 96 PCR扩增仪(Analytik Jena Corp., 德国)完成。PCR扩增产物经过纯化后,安装如上扩增方法完成8个循环的index扩增反应,每个样本的index标签不重复。将产物进行纯化后,按照等摩尔比进行混合,并采用Illumina Miseq平台(Illumina Inc., 美国)根据官方说明书操作完成测序(每个粪便样本至少完成2万条测序数据)。
1.6 伦理学审查
本研究方案经由西京医院药物临床试验伦理委员会审批,批号:KY20172061-1,患者均签署知情同意书。
1.7 统计学方法
分析SPSS 19.0和R v3.6.1统计软件进行数据分析。计量资料采用M(P25~P75)表示, 3组间比较采用Kruskal-Wallis H检验,采用Bonferroni法进行多个样本的多重比较;同组患者术前术后的比较采用Wilcoxo符号秩检验。计数资料组间比较采用χ2检验。微生物组Beta分析基于Bray Curtis距离矩阵进行主坐标分析(PCoA),使用Adonis法(PerMANOVA)对比组间差异。P<0.05为差异有统计学意义。
2. 结果
2.1 一般资料
共纳入接受TIPS治疗的MHE患者28例,术前患者中位年龄51岁,男13例,女15例,中位MELD评分13分,Child-Pugh分级A/B/C级分别9/15/4例。根据患者术后1个月内发生HE的类型分为3组:无HE组(n=8,28.6%)、MHE组(n=12,42.8%)和OHE组(n=8,28.6%)。3组患者术前临床资料均未见明显差异(P值均>0.05)(表 1)。
表 1 不同预后组术前基线特征比较指标 无HE组(n=8) MHE组(n=12) OHE组(n=8) χ2值 P值 年龄(岁) 49.5(45.8~52.8) 54.5(49.8~59.5) 46.5(44.3~52.8) 5.28 0.07 性别[男,例(%)] 3(37.5) 6(50.0) 4(50.0) 3.08 0.61 BMI(kg/m2) 18.7(18.3~20.7) 21.6(18.6~24.1) 21.4(19.6~23.8) 4.43 0.11 肝硬化病因[HBV,例(%)] 4(50.0) 7(58.3) 7(87.5) 7.39 0.35 MELD评分 10.9(7.8~13.2) 13.4(10.0~17.5) 13.4(12.7~14.9) 4.82 0.10 Child-Pugh分级[例(%)] 2.44 0.49 A(5~6) 4(50.0) 3(12.5) 2(25.0) B(7~9) 4(50.0) 6(50.0) 5(62.5) C(10~13) 0(0) 3(12.5) 1(12.5) INR 1.4(1.1~1.6) 1.4(1.2~1.9) 1.6(1.5~1.7) 4.92 0.10 血红蛋白(g/dl) 80.0(75.5~83.8) 87.0(79.3~94.3) 80.0(59.0~92.0) 1.69 0.43 总蛋白(mg/dl) 68.2(53.1~73.9) 68.0(55.7~78.9) 66.2(54.7~68.0) 0.83 0.66 Alb(g/dl) 36.5(30.6~39.3) 34.4(28.1~37.2) 32.7(31.4~35.5) 1.10 0.58 TBil(μmol/L) 15.2(10.6~20.6) 23.6(14.7~49.6) 28.1(20.4~36.1) 4.86 0.09 AST(U/L) 21.5(13.0~40.3) 21.0(11.3~30.0) 28.5(20.3~34.8) 1.77 0.41 ALT(U/L) 30.5(20.8~42.8) 27.5(21.0~42.5) 31.0(21.5~37.5) 0.01 0.99 ALP(U/L) 97.0(73.8~117.0) 65.5(49.8~92.8) 79.0(59.8~112.3) 3.69 0.16 γ-氨基丁酸(U/L) 32.0(21.0~45.8) 23.5(14.0~56.5) 31.0(20.0~49.0) 0.63 0.73 肌酐(mg/dl) 77.5(66.5~93.8) 85.5(73.0~105.0) 72.0(68.3~95.0) 1.46 0.48 静脉血氨(μg/dl) 51.0(21.0~60.0) 37.5(23.8~60.5) 36.0(27.5~47.5) 0.29 0.86 注:MELD,终末期肝病模型。 2.2 物种组成分析
总体患者的肠道菌群主要由5个优势菌门(平均相对丰度占比>1%)组成:厚壁菌门(45.0%)、拟杆菌门(30.6%)、变形菌门(17.2%)、放线菌门(4.78%)和疣微菌门(1.9%)。菌门水平3组间比较无明显差异,且术后未见明显改变(P值均>0.05)。
2.3 Alpha多样性指数分析
Alpha多样性包括Chao1指数、Ace指数、Shannon指数和Simpson指数,对比发现,术前3组患者的alpha多样性无明显差异(P值均>0.05)(表 2),术后各组alpha多样性无明显改变且3组间无明显差异(P值均>0.05)。
表 2 不同预后组术前alpha多样性比较多样性指数 无HE组(n=8) MHE组(n=12) OHE组(n=8) χ2值 P值 Ace指数 186.70(137.96~232.29) 214.69(144.20~354.14) 186.68(130.79~241.34) 0.96 0.62 Chao1指数 194.64(133.60~215.46) 203.40(138.58~352.64) 183.79(118.53~208.50) 1.03 0.60 Shannon指数 2.65(2.02~3.12) 2.95(2.56~3.95) 2.67(2.04~3.50) 1.71 0.43 Simpson指数 0.16(0.09~0.23) 0.09(0.05~0.16) 0.13(0.07~0.26) 2.46 0.29 2.4 Beta多样性指数分析
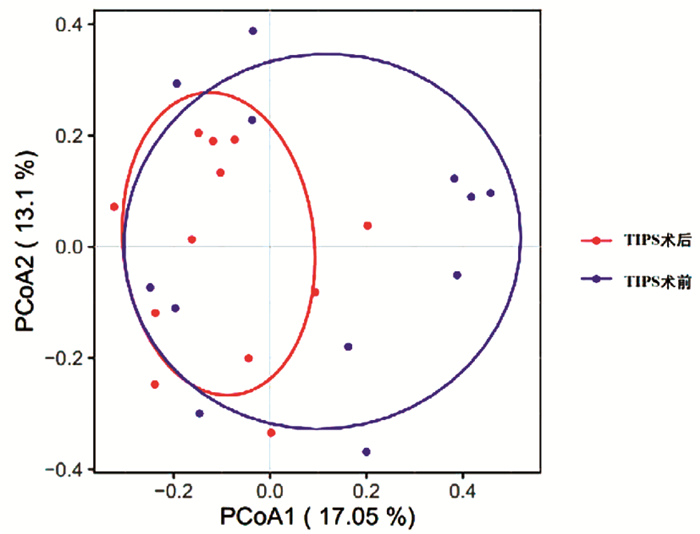
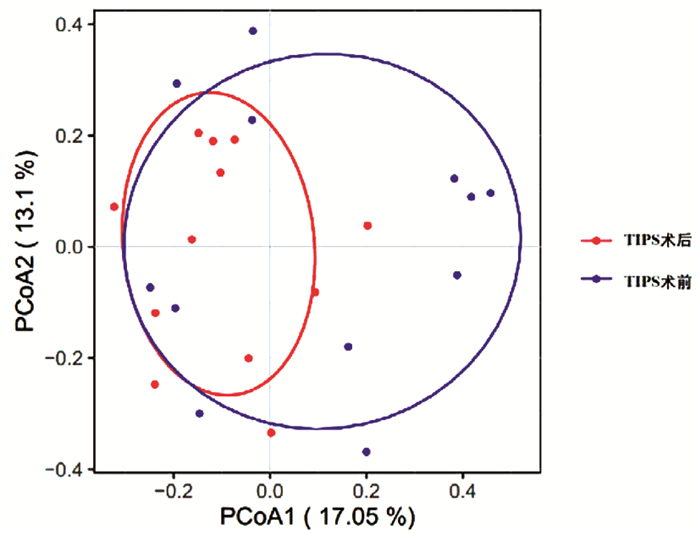
基于Bray Curtis距离矩阵的PCoA分析显示,3组间beta多样性在术前和术后没有明显差异。纵向对比中,仅MHE组术前和术后的beta多样性明显改变(PerMANOVA法,F=2.71,P=0.049)(图 1)。
2.5 物种差异分析
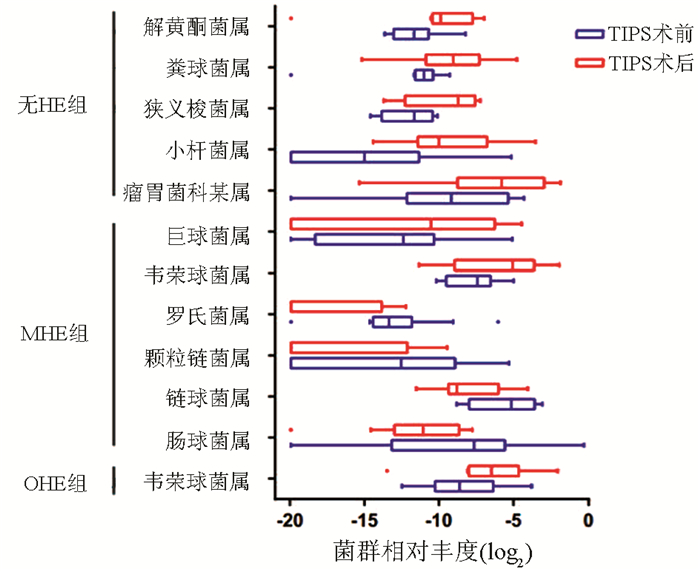
无HE组患者TIPS术后相比术前,原生菌群小杆菌属(Z=2.521,P=0.012)、粪球菌属(Z=2.1,P=0.036)、解黄酮菌属(Z=2.1,P=0.036)、瘤胃菌科某属(Z=2.1,P=0.036)和狭义梭菌属(Z=1.96,P=0.049)相对丰度显著升高。原生菌假丁酸弧菌属(Z=1.859,P=0.063)、有害菌如丹毒丝菌科未知属(Z=1.82,P=0.069)和颗粒链菌属(Z=1.82,P=0.069)相对平度差异均无统计学意义。
MHE组患者术后部分有害菌颗粒链菌属(Z=2.521,P=0.012)、肠球菌属(Z=2.51,P=0.012)、链球菌属(Z=2.432,P=0.015)和罗氏菌属(Z=2.001,P=0.045)丰度明显减低,而部分有害菌韦荣球菌属(Z=2.353,P=0.019)和巨球菌属(Z=1.955,P=0.05)则明显升高。
对于OHE组患者,仅有韦荣球菌属(Z=2.38,P=0.017)丰度显著升高,其他菌群丰度未见明显变化(图 2)。
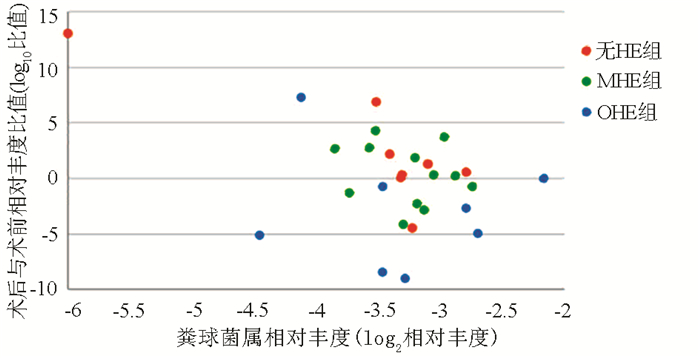
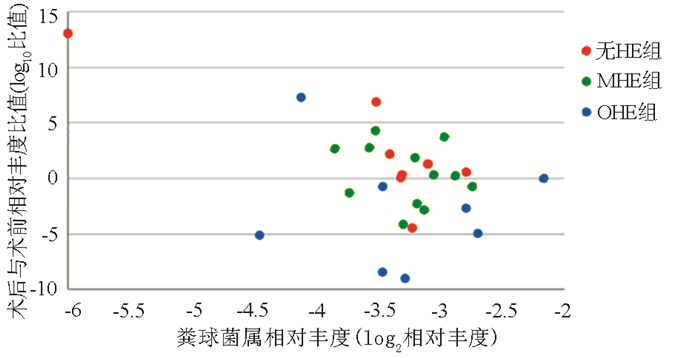
采用增长倍数(术后菌群相对丰度/术前菌群相对丰度,丰度为0则按10-6计算)来描述菌群术后的变化量,对比发现,粪球菌属的变化量在3组间具有明显差异[无HE组vs MHE组vs OHE组:2.00(1.11~91.61) vs 1.21(0.26~6.79) vs 0.09(0.01~0.92),χ2=6.249,P=0.043](图 3)。
3. 讨论
本研究通过16S rRNA测序的方法,开展了一项纵向观察性研究。描述MHE患者经TIPS术后不同脑病状态的患者肠道菌群的变化,探索术后菌群的改变与术后脑病程度关系。
TIPS术后,3组患者肠道菌群的alpha多样性无明显差异,总体菌群组成相似,可能与肝硬化失代偿期患者较差的菌群状态有关。术后无HE组患者,菌群失调好转,小杆菌属、粪球菌属、解黄酮菌属、瘤胃菌科某属、狭义梭菌属和假丁酸弧菌属增多,这些菌属多属于毛螺菌科和瘤胃菌科,是健康人体内的原生菌群,它们参与人肠道内碳水化合物发酵产生短链脂肪酸,减轻结肠炎症[12-15],且与致病菌竞争营养,产生抗菌肽,可能改善肠道屏障[16-18]。这在克罗恩病和肝硬化的研究中均有所体现[17-18]。而有害菌如丹毒丝菌科未知属和颗粒链菌属减少。
对于MHE组患者,颗粒链菌属、肠球菌属、链球菌属和罗氏菌属丰度减低,而韦荣球菌属和巨球菌属(均属韦荣球菌科下级菌属)则升高。而OHE组患者仅有韦荣球菌属增多。颗粒链菌属和罗氏菌属是口腔和肠道菌群中的机会致病菌,肠球菌属在肝硬化患者和HE患者中富集,与MELD评分有显著的正相关性[8-9]。链球菌属和韦荣球菌属与肠道菌群失调以及较差的认知水平相关[19-20]。Bajaj[9]报道了与健康人相比肝硬化HE患者韦荣球菌科相对丰度明显更高。另外,既往文献[21-23]发现韦荣球菌属某些菌种参与了数种严重的炎症反应。
因此本研究推测,虽然TIPS术建立了新的门体分流道,使部分肠源性毒素可以直接从门静脉进入体循环,但是术后门静脉高压得到缓解,肠道微循环和肠道环境发生改变,从而对肠道菌群产生了一定的影响。(1)原生菌群丰度升高而有害菌丰度降低,肠道微生态恢复,产生的肠源性毒素减少,MHE患者的脑病状态得到改善;(2)有害菌/潜在致病菌有的增多有的减少,菌群状态未得到明显改善,隐性脑病状态未缓解;(3)脑病富集菌韦荣球菌属明显增多,菌群失调加重,患者发生OHE。同时本研究发现粪球菌属的变化量与术后脑病的严重程度呈负相关。说明了原生菌群的增多对TIPS术后患者预后有更为重要的影响。
本研究存在一定的局限性。首先样本量较小,需要大样本多中心研究对结果进行验证;第二,使用16S rRNA测序而不是宏基因组使研究不能深入到菌种水平进行分析;第三,研究未考虑环境因素、饮食以及药物使用如抗生素和质子泵抑制剂等的影响;同时,TIPS术对肠道微环境具体产生了什么样的作用,为什么会出现不同的肠道菌群改变,以及对于发生HE的患者,是菌群恶化造成脑病发生还是脑病发生导致菌群恶化仍需要进一步研究。
总的来说,本研究报道了经TIPS术后不同脑病状态的MHE患者肠道菌群的变化情况,发现肠道菌群失调状态的改善情况与术后脑病状态呈负相关。其中原生菌群的升高相对有更重要的作用。这为预防和治疗MHE患者术后脑病提供了微生物角度策略。
-
表 1 不同预后组术前基线特征比较
指标 无HE组(n=8) MHE组(n=12) OHE组(n=8) χ2值 P值 年龄(岁) 49.5(45.8~52.8) 54.5(49.8~59.5) 46.5(44.3~52.8) 5.28 0.07 性别[男,例(%)] 3(37.5) 6(50.0) 4(50.0) 3.08 0.61 BMI(kg/m2) 18.7(18.3~20.7) 21.6(18.6~24.1) 21.4(19.6~23.8) 4.43 0.11 肝硬化病因[HBV,例(%)] 4(50.0) 7(58.3) 7(87.5) 7.39 0.35 MELD评分 10.9(7.8~13.2) 13.4(10.0~17.5) 13.4(12.7~14.9) 4.82 0.10 Child-Pugh分级[例(%)] 2.44 0.49 A(5~6) 4(50.0) 3(12.5) 2(25.0) B(7~9) 4(50.0) 6(50.0) 5(62.5) C(10~13) 0(0) 3(12.5) 1(12.5) INR 1.4(1.1~1.6) 1.4(1.2~1.9) 1.6(1.5~1.7) 4.92 0.10 血红蛋白(g/dl) 80.0(75.5~83.8) 87.0(79.3~94.3) 80.0(59.0~92.0) 1.69 0.43 总蛋白(mg/dl) 68.2(53.1~73.9) 68.0(55.7~78.9) 66.2(54.7~68.0) 0.83 0.66 Alb(g/dl) 36.5(30.6~39.3) 34.4(28.1~37.2) 32.7(31.4~35.5) 1.10 0.58 TBil(μmol/L) 15.2(10.6~20.6) 23.6(14.7~49.6) 28.1(20.4~36.1) 4.86 0.09 AST(U/L) 21.5(13.0~40.3) 21.0(11.3~30.0) 28.5(20.3~34.8) 1.77 0.41 ALT(U/L) 30.5(20.8~42.8) 27.5(21.0~42.5) 31.0(21.5~37.5) 0.01 0.99 ALP(U/L) 97.0(73.8~117.0) 65.5(49.8~92.8) 79.0(59.8~112.3) 3.69 0.16 γ-氨基丁酸(U/L) 32.0(21.0~45.8) 23.5(14.0~56.5) 31.0(20.0~49.0) 0.63 0.73 肌酐(mg/dl) 77.5(66.5~93.8) 85.5(73.0~105.0) 72.0(68.3~95.0) 1.46 0.48 静脉血氨(μg/dl) 51.0(21.0~60.0) 37.5(23.8~60.5) 36.0(27.5~47.5) 0.29 0.86 注:MELD,终末期肝病模型。 表 2 不同预后组术前alpha多样性比较
多样性指数 无HE组(n=8) MHE组(n=12) OHE组(n=8) χ2值 P值 Ace指数 186.70(137.96~232.29) 214.69(144.20~354.14) 186.68(130.79~241.34) 0.96 0.62 Chao1指数 194.64(133.60~215.46) 203.40(138.58~352.64) 183.79(118.53~208.50) 1.03 0.60 Shannon指数 2.65(2.02~3.12) 2.95(2.56~3.95) 2.67(2.04~3.50) 1.71 0.43 Simpson指数 0.16(0.09~0.23) 0.09(0.05~0.16) 0.13(0.07~0.26) 2.46 0.29 -
[1] European Association for the Study of the Liver. EASL clinical practice guidelines for the management of patients with decompensated cirrhosis[J]. J Hepatol, 2018, 69(2): 406-460. DOI: 10.1016/j.jhep.2018.03.024 [2] ZHANG JC, WANG YG, LIN F, et al. Recent advances on the pathogenesis of hepatic encephalopathy[J/CD]. Chin J Liver Dis (Electronic Version), 2019, 11(1): 6-11. (in Chinese)张军昌, 王永刚, 林芳, 等. 肝性脑病发病机制新进展[J/CD]. 中国肝脏病杂志(电子版), 2019, 11(1): 6-11. [3] RIDOLA L, CARDINALE V, RIGGIO O. The burden of minimal hepatic encephalopathy: From diagnosis to therapeutic strategies[J]. Ann Gastroenterol, 2018, 31(2): 151-164. [4] WANG JY, ZHANG NP, CHI BR, et al. Prevalence of minimal hepatic encephalopathy and quality of life evaluations in hospitalized cirrhotic patients in China[J]. World J Gastroenterol, 2013, 19(30): 4984-4991. DOI: 10.3748/wjg.v19.i30.4984 [5] QIN N, YANG F, LI A, et al. Alterations of the human gut microbiome in liver cirrhosis[J]. Nature, 2014, 513(7516): 59-64. DOI: 10.1038/nature13568 [6] TANG SH, CHEN H, HAN GH. Association between gut microbiota and hepatic encephalopathy in patients with liver cirrhosis[J]. J Clin Hepatol, 2019, 35(5): 1109-1113. (in Chinese) DOI: 10.3969/j.issn.1001-5256.2019.05.041汤世豪, 陈辉, 韩国宏. 肠道菌群与肝硬化肝性脑病的关系[J]. 临床肝胆病杂志, 2019, 35(5): 1109-1113. DOI: 10.3969/j.issn.1001-5256.2019.05.041 [7] LU BJ, ZHAO YH, AN YT, et al. Research advances in gut microbiota in liver cirrhosis and related complications[J]. J Clin Hepatol, 2018, 34(11): 2433-2437. (in Chinese) DOI: 10.3969/j.issn.1001-5256.2018.11.037鲁冰洁, 赵亚红, 安泳潼, 等. 肠道微生物在肝硬化及相关并发症中的研究进展[J]. 临床肝胆病杂志, 2018, 34(11): 2433-2437. DOI: 10.3969/j.issn.1001-5256.2018.11.037 [8] BAJAJ JS, HEUMAN DM, HYLEMON PB, et al. Altered profile of human gut microbiome is associated with cirrhosis and its complications[J]. J Hepatol, 2014, 60(5): 940-947. DOI: 10.1016/j.jhep.2013.12.019 [9] BAJAJ JS. The role of microbiota in hepatic encephalopathy[J]. Gut Microbes, 2014, 5(3): 397-403. DOI: 10.4161/gmic.28684 [10] Chinese Society of Hepatology, Chinese Medical Association. Guidelines on the management of hepatic encephalopathy in cirrhosis[J]. J Clin Hepatol, 2018, 34(10): 2076-2089. (in Chinese) DOI: 10.3969/j.issn.1001-5256.2018.10.007中华医学会肝病分学会. 肝硬化肝性脑病诊疗指南[J]. 临床肝胆病杂志, 2018, 34(10): 2076-2089. DOI: 10.3969/j.issn.1001-5256.2018.10.007 [11] FERENCI P. Hepatic encephalopathy-Definition, nomenclature, diagnosis, and quantification: Final report of the Working Party at the 11th World Congresses of Gastroenterology, Vienna, 1998[J]. Hepatology, 2002, 35(3): 716-721. DOI: 10.1053/jhep.2002.31250 [12] ZOETENDAL EG, BEN-AMOR K, HARMSEN HJ, et al. Quantification of uncultured Ruminococcus obeum-like bacteria in human fecal samples by fluorescent in situ hybridization and flow cytometry using 16S rRNA-targeted probes[J]. Appl Environ Microbiol, 2002, 68(9): 4225-4232. DOI: 10.1128/AEM.68.9.4225-4232.2002 [13] DABARD J, BRIDONNEAU C, PHILLIPE C, et al. Ruminococcin A, a new lantibiotic produced by a Ruminococcus gnavus strain isolated from human feces[J]. Appl Environ Microbiol, 2001, 67(9): 4111-4118. DOI: 10.1128/AEM.67.9.4111-4118.2001 [14] DUNCAN SH, LOUIS P, FLINT HJ. Cultivable bacterial diversity from the human colon[J]. Lett Appl Microbiol, 2007, 44(4): 343-350. DOI: 10.1111/j.1472-765X.2007.02129.x [15] WONG JM, de SOUZA R, KENDALL CW, et al. Colonic health: Fermentation and short chain fatty acids[J]. J Clin Gastroenterol, 2006, 40(3): 235-243. DOI: 10.1097/00004836-200603000-00015 [16] NAVA GM, STAPPENBECK TS. Diversity of the autochthonous colonic microbiota[J]. Gut Microbes, 2011, 2(2): 99-104. DOI: 10.4161/gmic.2.2.15416 [17] SOKOL H, PIGNEUR B, WATTERLOT L, et al. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients[J]. Proc Natl Acad Sci U S A, 2008, 105(43): 16731-16736. DOI: 10.1073/pnas.0804812105 [18] SOKOL H, LAY C, SEKSIK P, et al. Analysis of bacterial bowel communities of IBD patients: What has it revealed?[J]. Inflamm Bowel Dis, 2008, 14(6): 858-867. DOI: 10.1002/ibd.20392 [19] BAJAJ JS, BETRAPALLY NS, HYLEMON PB, et al. Salivary microbiota reflects changes in gut microbiota in cirrhosis with hepatic encephalopathy[J]. Hepatology, 2015, 62(4): 1260-1271. DOI: 10.1002/hep.27819 [20] BAJAJ JS, RIDLON JM, HYLEMON PB, et al. Linkage of gut microbiome with cognition in hepatic encephalopathy[J]. Am J Physiol Gastrointest Liver Physiol, 2012, 302(1): g168-g175. DOI: 10.1152/ajpgi.00190.2011 [21] de CRUZ P, KANG S, WAGNER J, et al. Association between specific mucosa-associated microbiota in Crohn's disease at the time of resection and subsequent disease recurrence: A pilot study[J]. J Gastroenterol Hepatol, 2015, 30(2): 268-278. DOI: 10.1111/jgh.12694 [22] BONGAERTS GP, SCHREURS BW, LUNEL FV, et al. Was isolation of Veillonella from spinal osteomyelitis possible due to poor tissue perfusion?[J]. Med Hypotheses, 2004, 63(4): 659-661. DOI: 10.1016/j.mehy.2004.02.052 [23] ROVERY C, ETIENNE A, FOUCAULT C, et al. Veillonella montpellierensis endocarditis[J]. Emerg Infect Dis, 2005, 11(7): 1112-1114. DOI: 10.3201/eid1107.041361 期刊类型引用(3)
1. 王文青,张樱,黄人鹤,李斌华,吴军,李丽,闫雪华. 肝硬化门静脉高压患者TIPS相关肝性脑病与肠道微生物相关性研究进展. 中国介入影像与治疗学. 2024(10): 624-627 .  百度学术
百度学术2. 徐华栋,张玉婷. 亚临床肝性脑病患者肠道菌群和炎症因子水平变化及黄连素的干预作用. 中国医学创新. 2023(27): 141-145 .  百度学术
百度学术3. 黄婷萍,王广川,黄广军,张春清. 基于机器学习算法的肝硬化患者经颈静脉肝内门体分流术后临床结局预测. 中华消化病与影像杂志(电子版). 2022(01): 4-10 .  百度学术
百度学术其他类型引用(1)
-




 PDF下载 ( 2149 KB)
PDF下载 ( 2149 KB)

 下载:
下载:



 下载:
下载:

 百度学术
百度学术