不同细胞病理学分级标准对超声内镜引导下细针穿刺诊断胰腺癌的影响
DOI: 10.3969/j.issn.1001-5256.2021.02.028
Effect of different cytopathological grading standards on the diagnosis of pancreatic cancer by endoscopic ultrasound-guided fine needle aspiration
-
摘要:
目的 探讨不同细胞病理学分级标准对超声内镜引导下细针穿刺(EUS-FNA)诊断胰腺癌效能的影响。 方法 收集2011年5月—2019年3月于安徽医科大学第一附属医院行EUS-FNA检查的256例胰腺占位患者的临床资料和胰腺细胞病理学诊断结果,以手术病理结果和随访情况作为最终诊断,评估影响EUS-FNA诊断效能的相关因素。计量资料两组间比较采用独立样本t检验或Mann-Whitney U检验;计数资料两组间比较采用χ2检验。应用受试者工作特征曲线(ROC曲线)评价不同细胞病理学分级标准对胰腺癌的诊断价值。 结果 剔除失访患者67例,共189例患者纳入研究,按巴氏细胞病理学标准,EUS-FNA诊断细胞病理学结果为异型细胞47例,疑癌细胞25例,癌细胞20例,未见肿瘤细胞97例。133例经术后病理和随访结果证实为胰腺癌,其中细胞病理学检查结果分别为:未见肿瘤细胞52例,异型细胞36例,疑癌细胞25例,癌细胞20例。EUS-FNA诊断胰腺癌的真阳性率为60.90%(81例),假阴性率为39.10%(52例);非胰腺癌56例,假阳性率为19.64%(11例),真阴性率为80.36%(45例)。EUS-FNA诊断胰腺癌的ROC曲线下面积为0.643(95% CI:0.561~0.724)。联合不同细胞病理学分级标准,分别以“发现异型细胞或可疑癌细胞或癌细胞均为阳性”“发现可疑癌细胞或癌细胞均为阳性”和“发现癌细胞为阳性”为诊断标准进行分析,结果显示,以“发现异型细胞或可疑癌细胞或癌细胞均为阳性”为诊断标准,EUS-FNA诊断胰腺癌的效能提高,敏感度为50.38%,特异度为75.00%。189例患者EUS-FNA术后并发症发生率为6.88%(13例),主要为高淀粉酶血症和腹痛。 结论 联合不同细胞病理学分级标准有助于提高EUS-FNA对胰腺癌的诊断效能。 -
关键词:
- 胰腺肿瘤 /
- 内镜超声引导细针穿刺 /
- 诊断
Abstract:Objective To investigate the effect of different cytopathological grading standards on the efficiency of endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) in the diagnosis of pancreatic cancer. Methods Related clinical data and pancreatic cytopathological results were collected from 256 patients with pancreatic space-occupying lesions who underwent EUS-FNA in The First Affiliated Hospital of Anhui Medical University from May 2011 to March 2019, and the influencing factors for the diagnostic efficiency of EUS-FNA were analyzed based on surgical pathology and follow-up results. The independent samples t-test or the Mann-Whitney U test was used for comparison of continuous data between two groups, and the chi-square test was used for comparison of categorical data between two groups. The receiver operating characteristic (ROC) curve was used to evaluate the value of different cytopathological grading standards in the diagnosis of pancreatic cancer. Results A total of 67 patients who were lost to follow-up were excluded, and a total of 189 patients were included in the study. According to the Papanicolaou cytopathological standard, there were 47 cases of heterotypic cells, 25 cases of suspected cancer cells, 20 cases of cancer cells, and 97 cases without tumor cells based on EUS-FNA. A total of 133 patients were confirmed to have pancreatic cancer by postoperative pathology and follow-up results, among whom 52 had no tumor cells, 36 had heterotypic cells, 25 had suspected cancer cells, and 20 had cancer cells based on cytopathological results. EUS-FNA had a true positive rate of 60.90% (81 patients) and a false negative rate of 39.10% (52 patients) in the diagnosis of pancreatic cancer; for the 56 patients without pancreatic cancer, EUS-FNA had a false positive rate of 19.64% (11 patients) and a true negative rate of 80.36% (45 patients). EUS-FNA had an area under the ROC curve of 0.643 (95% confidence interval: 0.561-0.724) in the diagnosis of pancreatic cancer. In combination with different cytopathological grading standards and with the diagnostic criteria of "the identification of heterotypic cells or suspected cancer cells or cancer cells was considered positive", "the identification of suspected cancer cells or cancer cells was considered positive", and "the identification of cancer cells was considered positive", the results showed that the diagnostic criteria of "the identification of heterotypic cells or suspected cancer cells or cancer cells was considered positive" improved the efficiency of EUS-FNA in the diagnosis of pancreatic cancer, with a sensitivity of 50.38% and a specificity of 75.00%. Among the 189 patients, 13 (6.88%) experienced complications after EUS-FNA, which included hyperamylasemia and abdominal pain. Conclusion The combination of different cytopathological grading standards can help improve the efficiency of EUS-FNA in the diagnosis of pancreatic cancer. -
由于胰腺解剖位置特殊,胰腺癌患者早期多无症状,一经发现已进入中晚期,外科手术是胰腺癌的有效治疗方法。但外科手术创伤大,术后生活质量差,因此早期诊断具有重要意义。胰腺癌诊断方法包括CT、MRI等影像学检查,有助于精确分级和确定可切除状态,但此类检查方法敏感度不高,早期胰腺癌检出率较低,同时难以获得病理学诊断[1-2]。超声内镜(EUS)是近年来用于胰腺病变检查的重要技术,不仅可以获得近距离的高分辨率图像,同时可以通过超声内镜引导下细针穿刺(EUS-FNA)获得细胞病理学结果,明确胰腺病变性质[3-4]。EUS-FNA可以显著提高胰腺癌诊断的准确性,具有较好的敏感度和特异度[5]。Eltoum等[6]研究发现,自引入EUS-FNA技术以来,通过获取胰腺细胞病理学方法诊断胰腺病变的病例数越来越多,依靠细胞学诊断结果进行后续治疗的胰腺病变比例从19%上升至51%;同时未进行细胞学检查,仅依靠获取胰腺手术病理诊断胰腺病变的比例从56%降低到23%。不同细胞病理学诊断标准可能影响EUS-FNA诊断胰腺肿瘤的准确程度[7]。Hewitt等[8]对胰腺病变EUS-FNA进行荟萃分析显示,EUS-FNA诊断胰腺肿瘤具有较高的敏感度和特异度。当采用癌细胞作为阳性结果时,敏感度为85%,特异度为98%;当采用异型细胞、疑癌细胞和癌细胞作为阳性结果时,合并敏感度为91%,合并特异度为94%。因内镜医师操作和病理医师诊断水平差异的影响,提高EUS-FNA对于胰腺占位性病变诊断的准确率是目前研究的热点[7]。因此,本研究通过收集胰腺占位患者EUS-FNA的临床资料并进行相关分析,采用不同细胞病理学诊断标准,探讨EUS-FNA诊断胰腺癌的临床效能。
1. 资料与方法
1.1 研究对象
选取2011年5月—2019年3月本院收治并进行EUS-FNA检查的胰腺占位患者。记录患者一般资料,包括性别、年龄、吸烟史、饮酒史、糖尿病、胰腺炎病史、临床表现、肝功能和肿瘤标志物等;EUS、CT等影像学资料以及EUS-FNA细胞病理学结果。随访时间为1年以上。
1.2 纳入标准与排除标准
纳入标准:(1)患者以腹痛、黄疸、乏力等症状就诊,疑诊胰腺占位病变;(2)患者均行EUS-FNA细胞学检查;(3)所有患者签署相关知情同意书;(4)患者术前均无胰腺手术或放化疗治疗史。排除标准:(1)EUS检查禁忌证;(2)合并严重心、肺功能不全,难以耐受EUS-FNA检查;(3)合并严重凝血功能障碍;(4)合并精神和意识障碍;(5)EUS-FNA获取的胰腺标本量不充分,不足以进行病理学诊断。
1.3 方法
1.3.1 EUS检查
所有患者行EUS术前均完善常规检查,术前禁食12 h、禁水4 h。部分患者采用丙泊酚联合芬太尼静脉推注麻醉;部分患者术前30 min肌注哌替啶50 mg、地西泮10 mg。常规进镜明确病变位置,调整EUS探头显示胰腺病变,记录胰腺病变部位、形态、回声、大小、边界,胰管和胆总管是否扩张,周围淋巴结,以及病变与血管的毗邻关系等。
1.3.2 EUS-FNA检查
所有EUS-FNA操作均由消化内科专职医师完成。EUS定位扫查胰腺病灶,注意避开血管,选择合适进针角度及深度,超声实时监测下完成EUS-FNA。所用穿刺针为美国Wilson-Cook Medical生产的ECHO 19/22G穿刺针。将穿刺针穿刺至病灶内,拔除针芯,连接10 ml负压注射器常规穿刺。观察穿刺点出血停止后退镜。穿刺针所得组织涂片经HE染色后细胞学检查。患者常规禁食24 h,监测生命体征。由病理科专职医师完成细胞病理学检查,细胞病理学诊断标准采用巴氏标准[5]:(1)癌细胞;(2)疑癌细胞;(3)异型细胞;(4)未见肿瘤细胞(包括炎细胞和正常上皮细胞等)。
1.4 诊断标准
行手术治疗患者,最终诊断以手术病理结果为准。未行手术治疗,相关辅助检查(如PET-CT、CA19-9、CEA)等提示胰腺癌并且随访期间死亡的患者,最终诊断为胰腺癌[9-10]; 未行手术治疗并且随访期间存活的患者,最终诊断为非胰腺癌。
1.5 伦理学审查
本研究方案经由安徽医科大学第一附属医院伦理委员会审批,批号:PJ2018-12-17,所有患者均签署知情同意书。
1.6 统计学方法
采用SPSS 22.0统计软件进行数据分析。正态分布的计量资料用x ± s表示,两组间比较采用独立样本t检验;非正态分布的计量资料用M(P25~P75)表示,两组间比较采用Mann-Whitney U检验。计数资料两组间比较采用χ2检验。应用受试者工作特征曲线(ROC曲线)评价不同细胞病理学分级标准对胰腺癌的诊断价值。P<0.05为差异有统计学意义。
2. 结果
2.1 一般资料
共收治胰腺占位患者256例,剔除失访67例,最终纳入研究189例,按巴氏细胞病理学标准,EUS-FNA诊断细胞病理学结果为异型细胞47例,疑癌细胞25例,癌细胞20例,未见肿瘤细胞97例。其中133例为胰腺癌(胰腺癌组),包括经术后病理证实者22例和临床检查结果为胰腺癌且随访期间死亡者111例;56例为非胰腺癌(非胰腺癌组)。两组患者年龄、发生胰腺炎比例、PLT、DBil、GGT、CA19-9、CEA比较,差异均有统计学意义(P值均<0.05)(表 1)。
表 1 胰腺癌与非胰腺癌组患者的临床特征比较指标 非胰腺癌组(n=56) 胰腺癌组(n=133) 统计值 P值 男[例(%)] 32(57.1) 85(63.9) χ2=0.765 0.382 年龄(岁) 54.9±14.3 63.3±11.4 t=4.257 <0.001 非甾体抗炎药用药史[例(%)] 1(1.8) 12(9.0) χ2=2.191 0.139 糖尿病史[例(%)] 5(8.9) 17(12.8) χ2=0.569 0.451 胰腺炎病史[例(%)] 14(25.0) 12(9.0) χ2=8.479 0.004 WBC(109/L) 6.02±2.08 5.72±1.95 t=0.924 0.357 RBC(1012/L) 4.32±0.64 4.16±0.63 t=1.588 0.114 Hb(g/L) 124.16±20.47 122.11±17.94 t=0.686 0.494 PLT(109/L) 214.61±88.11 186.88±80.94 t=2.086 0.038 血清Ca2+(mmol/L) 2.28±0.12 2.27±0.18 t=0.158 0.875 肌酐(μmol/L) 60.62±14.50 18.05±1.64 t=1.431 0.154 尿素氮(mmol/L) 5.30(4.31~6.05) 5.23(3.83~6.49) U=1497.50 0.858 Alb(g/L) 40.30±4.76 40.69±0.57 t=0.414 0.679 TBil(μmol/L) 13.36(10.81~14.62) 19.85(12.15~29.78) U=1668.00 0.081 DBil(μmol/L) 2.72(2.04~3.60) 4.24(3.76~16.86) U=1588.00 <0.001 ALT(U/L) 16.00(14.00~18.00) 26.50(14.50~138.50) U=1819.50 0.072 AST(U/L) 19.00(15.00~24.00) 27.50(17.00~74.00) U=1792.50 0.117 GGT(U/L) 22.00(19.00~29.00) 48.00(22.00~315.50) U=1591.00 0.002 ALP(U/L) 103.00(95.00~127.00) 115.00(66.00~347.50) U=3070.00 0.266 CRP(mg/L) 4.74(0.79~62.18) 4.02(1.52~18.08) U=638.00 0.827 Glu(mmol/L) 6.40±3.07 6.57±1.97 t=0.356 0.723 脂肪酶(U/L) 43.00(25.00~80.00) 51.00(24.00~329.00) U=501.50 0.106 淀粉酶(U/L) 72.00(61.00~80.00) 67.50(39.00~128.00) U=2166.00 0.223 CA19-9(U/L) 59.94(10.19~192.30) 141.00(30.03~298.80) U=1590.50 <0.001 CEA(mg/ml) 2.00(1.00~3.00) 3.00(2.00~5.00) U=1878.00 <0.001 22例胰腺癌组术后获得病理诊断的患者中胰腺导管腺癌16例(细胞学阳性11例,阴性5例),胰腺囊腺癌2例(细胞学均阳性),导管内乳头状黏液癌3例(细胞学阳性2例,阴性1例),乳头状囊腺癌1例(细胞学阴性)。其余167例患者未行手术治疗,其中放化疗63例,未做任何积极治疗104例。
胰腺癌组中假阴性患者共52例,其中年龄>70岁45例,肝功能损害和CA19-9升高不明显;年龄<70岁7例,肝功能损害和CA19-9升高明显,患者多因高龄体弱,拒绝放化疗及手术治疗。非胰腺癌组中假阳性11例,多有慢性胰腺炎病史,肝功能正常或轻度异常,CA19-9无明显升高等。
2.2 两组患者EUS特征比较
189例患者中,病变位于胰头部116例,胰体尾部73例。EUS显示为实性病变145例(76.7%),囊性/囊实性病变44例。胰腺癌患者的EUS多表现为胰腺不均质低回声占位,以实性包块为主,病变边界不规则,胰管扩张显著;非胰腺癌患者EUS多表现为胰腺囊性或囊实性病变,回声不均匀,病变边界相对清晰,胰管多不扩张(表 2)。
表 2 胰腺癌与非胰腺癌组患者EUS特征比较项目 非胰腺癌组(n=56) 胰腺癌组(n=133) χ2值 P值 病变部位(例) 0.015 0.904 胰头部 34 82 胰体尾部 22 51 胰管扩张[例(%)] 18(32.1) 70(52.6) 6.649 0.010 边界清晰[例(%)] 36(64.3) 34(25.6) 25.338 <0.001 性质(例) 6.888 0.009 囊性/囊实性 20 24 实性 36 109 低回声[例(%)] 43(76.8) 121(91.0) 6.915 0.009 2.3 采用不同细胞学分级标准对EUS-FNA胰腺癌诊断效能的影响
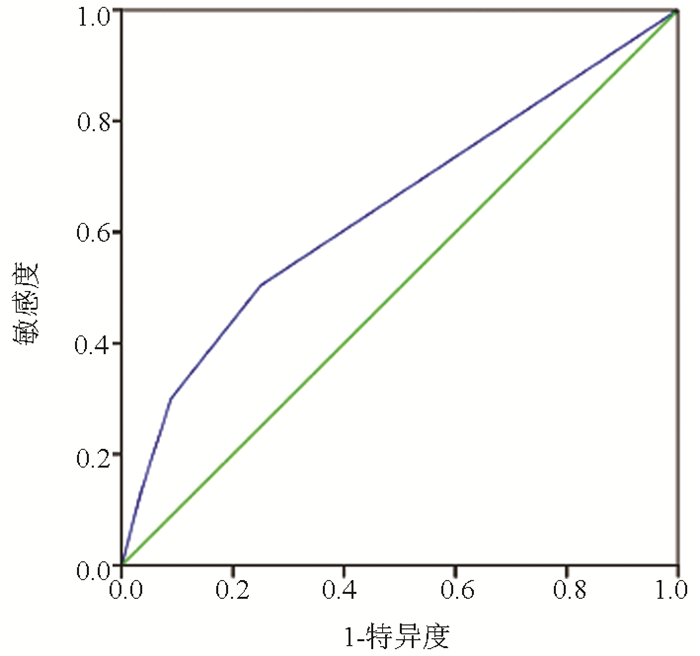
189例患者中胰腺癌患者133例,EUS-FNA细胞病理学检查结果分别为:未见肿瘤细胞52例,异型细胞36例,疑癌细胞25例,癌细胞20例。按照诊断金标准,EUS-FNA细胞病理学诊断胰腺癌效能结果如图 1所示,ROC曲线下面积(AUC)为0.643(95%CI:0.561~0.724)。
联合不同的细胞病理学分级标准,即分别以“发现异型细胞或可疑癌细胞或癌细胞均为阳性”“发现可疑癌细胞或癌细胞均为阳性”和“发现癌细胞为阳性”为诊断标准进行分析[6],结果显示,以“发现异型细胞或可疑癌细胞或癌细胞均为阳性”为诊断标准,EUS-FNA诊断胰腺癌的效能最显著,敏感度为50.38%,特异度为75.00%(表 3)。
表 3 EUS-FNA细胞学结果诊断胰腺癌效能分析EUS-FNA结果 AUC 敏感度(%) 特异度(%) Youden指数 Kappa(%) 阳性预测值(%) 阴性预测值(%) 假阴性率(%) 假阳性率(%) 发现异型细胞或可疑癌细胞或癌细胞均为阳性 0.643 50.38 75.00 0.25 20.00 82.71 38.89 49.62 25.00 发现可疑癌细胞或癌细胞均为阳性 0.606 30.08 91.07 0.21 14.53 88.89 35.42 69.92 8.92 发现癌细胞为阳性 0.550 13.53 96.43 0.10 6.29 90.00 31.95 86.47 3.57 2.4 EUS-FNA检查术后相关并发症
189例患者行EUS- FNA检查术后并发症发生率为6.88%(13例),其中7例出现血淀粉酶升高,予以对症治疗后好转,6例出现腹痛,未予以特殊处理,1~6 h后自行缓解;未出现发热、感染、出血、穿孔、重症胰腺炎等其他并发症。
3. 讨论
胰腺癌具有恶性程度高、起病隐匿、病情进展快、病死率高等特点,早期诊断是提高疗效的重要方法。在胰腺癌高危患者筛查中,除了肿瘤标志物和上腹部CT外,联合EUS检查可以显著提高诊断率、敏感度和特异度[8],尤其是小胰腺癌的EUS检查可与传统的影像学检查形成优势互补[11]。本研究结果表明,在临床特征方面,胰腺癌患者具有高龄、梗阻性黄疸以及发生胰腺炎比例较低等特点,EUS表现为胰腺不均质低回声占位,以实性包块为主,病变边界不规则,胰管扩张显著,机制上均与胰腺癌侵犯周围器官的占位效应有关。因此,对于EUS-FNA假阴性患者如具有上述临床和EUS特征,则需要高度怀疑胰腺癌,积极手术治疗。EUS-FNA是一项可以安全、准确获取病变标本并行病理诊断的技术,目前广泛用于胰腺肿瘤的定性诊断[11-12],对胰腺癌具有较高的敏感度和准确度,为诊断胰腺病变性质提供了新的思路[13]。与既往研究结果[14]类似。本研究结果显示,当以细胞病理学查见癌细胞为阳性结果时,EUS-FNA诊断胰腺癌的特异度为96.43%,但敏感度为13.53%。Hewitt等[8]进行Meta分析表明,以发现癌细胞为标准,假阴性率在5%~46%范围内波动,假阳性率在0~29%范围内波动,以发现异型细胞或可疑癌细胞或癌细胞为标准,则假阴性率降低到4%~32%,但假阳性上升到0~33%。Layfield等[7]研究表明,采用不同分级的细胞学诊断标准,即“肿瘤”“异型性”“可疑恶性”和“恶性”时,假阴性率分别为15.77%、20.72%、28.83%、37.39%,假阳性率分别为16.05%、12.35%、6.17%、2.47%,因此,采用不同病理标准EUS-FNA的假阴性率和假阳性率均有明显不同。本研究采用不同细胞学诊断标准,发现假阴性率分别为49.62%、69.92%、86.47%,假阳性率分别为25.00%、8.92%、3.57%。若采用癌细胞作为细胞学诊断标准,可能出现假阴性率过高的结果。
有研究[15-16]表明,EUS-FNA诊断胰腺病变总体敏感度为80%~90%,总体特异度高达90%~100%,诊断准确度>85%。病灶大小、部位及是否伴有血管侵犯等因素是影响EUS-FNA漏诊胰腺癌的重要因素[17]。尽管EUS-FNA诊断胰腺癌的准确性高,但在实际工作中需注意假阳性,并且降低假阴性率。EUS-FNA假阳性多见于慢性胰腺炎。研究[18]表明,EUS-FNA的假阳性病例主要是由于慢性胰腺炎背景下的细胞学错误解释所致。对于被炎性组织或纤维化组织所包裹的胰腺癌,在穿刺时由于局部病灶硬化,增加穿刺难度,影响胰腺癌诊断的敏感性[19]。对于EUS-FNA假阴性患者如临床高度怀疑胰腺癌,则仍需要积极手术治疗。假阴性主要是受穿刺部位选择不当以及穿刺前新辅助化疗等因素影响,标本处理不当同样影响EUS-FNA病理结果分析。标本影响因素包括取材量不足、标本处理和染色时间等,以及与细胞学医师进行诊断时比较谨慎有关。与国外研究比较,本研究中EUS-FNA诊断胰腺癌假阴性率过高,主要与细胞病理学诊断医师的临床经验和标准过严有关,还与内镜医生识别胰腺病变EUS图像的熟练程度、穿刺病变部位的选择和技术等因素有关。因此,加强EUS-FNA操作者与病理医生的沟通,可能有助于病理医生对EUS-FNA组织的分析判断。另一方面,细胞学医师需要综合细胞数量和具体病变的超声形态学改变对穿刺标本进行评估[20]。本研究提示,操作人员应熟知胰腺及周围组织的解剖关系,并熟练掌握EUS及穿刺技术,与细胞学医师紧密配合,同时联合不同的细胞学诊断标准,以降低假阴性率的发生,提高EUS-FNA的诊断效能。采用巴氏细胞学不同分级标准联合分析,结合患者临床表现、实验室检查结果及影像学结果,有助于提高EUS-FNA细胞病理学诊断胰腺癌的准确性和病情评估。
EUS-FNA总体并发症发生率和致死率低。常见的并发症主要包括胰腺炎、疼痛、出血、发热、感染、穿孔及肿瘤细胞针道种植等[21]。本研究中EUS-FNA并发症发生率为6.88%,提示EUS-FNA具有较高的操作安全性。
总之,EUS-FNA在临床诊断胰腺癌中具有准确性较好的优点,与细胞病理学医师协同有助于提高胰腺癌的诊断率。
-
表 1 胰腺癌与非胰腺癌组患者的临床特征比较
指标 非胰腺癌组(n=56) 胰腺癌组(n=133) 统计值 P值 男[例(%)] 32(57.1) 85(63.9) χ2=0.765 0.382 年龄(岁) 54.9±14.3 63.3±11.4 t=4.257 <0.001 非甾体抗炎药用药史[例(%)] 1(1.8) 12(9.0) χ2=2.191 0.139 糖尿病史[例(%)] 5(8.9) 17(12.8) χ2=0.569 0.451 胰腺炎病史[例(%)] 14(25.0) 12(9.0) χ2=8.479 0.004 WBC(109/L) 6.02±2.08 5.72±1.95 t=0.924 0.357 RBC(1012/L) 4.32±0.64 4.16±0.63 t=1.588 0.114 Hb(g/L) 124.16±20.47 122.11±17.94 t=0.686 0.494 PLT(109/L) 214.61±88.11 186.88±80.94 t=2.086 0.038 血清Ca2+(mmol/L) 2.28±0.12 2.27±0.18 t=0.158 0.875 肌酐(μmol/L) 60.62±14.50 18.05±1.64 t=1.431 0.154 尿素氮(mmol/L) 5.30(4.31~6.05) 5.23(3.83~6.49) U=1497.50 0.858 Alb(g/L) 40.30±4.76 40.69±0.57 t=0.414 0.679 TBil(μmol/L) 13.36(10.81~14.62) 19.85(12.15~29.78) U=1668.00 0.081 DBil(μmol/L) 2.72(2.04~3.60) 4.24(3.76~16.86) U=1588.00 <0.001 ALT(U/L) 16.00(14.00~18.00) 26.50(14.50~138.50) U=1819.50 0.072 AST(U/L) 19.00(15.00~24.00) 27.50(17.00~74.00) U=1792.50 0.117 GGT(U/L) 22.00(19.00~29.00) 48.00(22.00~315.50) U=1591.00 0.002 ALP(U/L) 103.00(95.00~127.00) 115.00(66.00~347.50) U=3070.00 0.266 CRP(mg/L) 4.74(0.79~62.18) 4.02(1.52~18.08) U=638.00 0.827 Glu(mmol/L) 6.40±3.07 6.57±1.97 t=0.356 0.723 脂肪酶(U/L) 43.00(25.00~80.00) 51.00(24.00~329.00) U=501.50 0.106 淀粉酶(U/L) 72.00(61.00~80.00) 67.50(39.00~128.00) U=2166.00 0.223 CA19-9(U/L) 59.94(10.19~192.30) 141.00(30.03~298.80) U=1590.50 <0.001 CEA(mg/ml) 2.00(1.00~3.00) 3.00(2.00~5.00) U=1878.00 <0.001 表 2 胰腺癌与非胰腺癌组患者EUS特征比较
项目 非胰腺癌组(n=56) 胰腺癌组(n=133) χ2值 P值 病变部位(例) 0.015 0.904 胰头部 34 82 胰体尾部 22 51 胰管扩张[例(%)] 18(32.1) 70(52.6) 6.649 0.010 边界清晰[例(%)] 36(64.3) 34(25.6) 25.338 <0.001 性质(例) 6.888 0.009 囊性/囊实性 20 24 实性 36 109 低回声[例(%)] 43(76.8) 121(91.0) 6.915 0.009 表 3 EUS-FNA细胞学结果诊断胰腺癌效能分析
EUS-FNA结果 AUC 敏感度(%) 特异度(%) Youden指数 Kappa(%) 阳性预测值(%) 阴性预测值(%) 假阴性率(%) 假阳性率(%) 发现异型细胞或可疑癌细胞或癌细胞均为阳性 0.643 50.38 75.00 0.25 20.00 82.71 38.89 49.62 25.00 发现可疑癌细胞或癌细胞均为阳性 0.606 30.08 91.07 0.21 14.53 88.89 35.42 69.92 8.92 发现癌细胞为阳性 0.550 13.53 96.43 0.10 6.29 90.00 31.95 86.47 3.57 -
[1] KANDEL P, WALLACE MB. Advanced EUS guided tissue acquisition methods for pancreatic cancer[J]. Cancers (Basel), 2018, 10(2): 54. DOI: 10.3390/cancers10020054 [2] JIN KZ, HUANG QY, LIU C, et al. New advances in diagnosis and treatment of pancreatic cancer[J]. Chin J Dig Surg, 2019, 18(7): 657-661. (in Chinese) DOI: 10.3760/cma.j.issn.1673-9752.2019.07.009金凯舟, 黄秋依, 刘辰, 等. 胰腺癌诊断与治疗的新进展[J]. 中华消化外科杂志, 2019, 18(7): 657-661. DOI: 10.3760/cma.j.issn.1673-9752.2019.07.009 [3] CAǦLAR E, HATEMI I, ATASOY D, et al. Concordance of endoscopic ultrasonography-guided fine needle aspiration diagnosis with the final diagnosis in subepithelial lesions[J]. Clin Endosc, 2013, 46(4): 379-383. DOI: 10.5946/ce.2013.46.4.379 [4] YANG F, YANG F, SUN SY. Endoscopic ultrasound diagnosis of space-occupying lesions in the pancreas[J]. J Clin Hepatol, 2020, 36(8): 1704-1709. (in Chinese) DOI: 10.3969/j.issn.1001-5256.2020.08.005杨帆, 杨飞, 孙思予. 胰腺占位性病变的超声内镜诊断[J]. 临床肝胆病杂志, 2020, 36(8): 1704-1709. DOI: 10.3969/j.issn.1001-5256.2020.08.005 [5] WANG W, SHPANER A, KRISHNA SG, et al. Use of EUS-FNA in diagnosing pancreatic neoplasm without a definitive mass on CT[J]. Gastrointest Endosc, 2013, 78(1): 73-80. DOI: 10.1016/j.gie.2013.01.040 [6] ELTOUM IA, ALSTON EA, ROBERSON J. Trends in pancreatic pathology practice before and after implementation of endoscopic ultrasound-guided fine-needle aspiration: An example of disruptive innovation effect?[J]. Arch Pathol Lab Med, 2012, 136(4): 447-453. DOI: 10.5858/arpa.2011-0218-OA [7] LAYFIELD LJ, DODD L, FACTOR R, et al. Malignancy risk associated with diagnostic categories defined by the Papanicolaou Society of Cytopathology pancreaticobiliary guidelines[J]. Cancer Cytopathol, 2014, 122(6): 420-427. DOI: 10.1002/cncy.21386 [8] HEWITT MJ, McPHAIL MJ, POSSAMAI L, et al. EUS-guided FNA for diagnosis of solid pancreatic neoplasms: A meta-analysis[J]. Gastrointest Endosc, 2012, 75(2): 319-331. DOI: 10.1016/j.gie.2011.08.049 [9] KIM SH, WOO YS, LEE KH, et al. Preoperative EUS-guided FNA: Effects on peritoneal recurrence and survival in patients with pancreatic cancer[J]. Gastrointest Endosc, 2018, 88(6): 926-934. DOI: 10.1016/j.gie.2018.06.024 [10] YAN H, JIA A, ZHANG J, et al. The diagnostic value of endoscopic ultrasonography-guided fine needle aspiration for pancreatic space-occupying diseases[J]. J Xi'an Jiaotong Univ(Med Sci), 2020, 41(3): 425-428. (in Chinese) https://www.cnki.com.cn/Article/CJFDTOTAL-XAYX202003021.htm严欢, 贾皑, 张娟, 等. 超声内镜引导细针穿刺活检对胰腺占位性疾病的诊断价值[J]. 西安交通大学学报(医学版), 2020, 41(3): 425-428. https://www.cnki.com.cn/Article/CJFDTOTAL-XAYX202003021.htm [11] KITANO M, YOSHIDA T, ITONAGA M, et al. Impact of endoscopic ultrasonography on diagnosis of pancreatic cancer[J]. J Gastroenterol, 2019, 54(1): 19-32. DOI: 10.1007/s00535-018-1519-2 [12] CAZACU IM, LUZURIAGA CHAVEZ AA, SAFTOIU A, et al. A quarter century of EUS-FNA: Progress, milestones, and future directions[J]. Endosc Ultrasound, 2018, 7(3): 141-160. DOI: 10.4103/eus.eus_19_18 [13] LEBLANC JK, EMERSON RE, DEWITT J, et al. A prospective study comparing rapid assessment of smears and ThinPrep for endoscopic ultrasound-guided fine-needle aspirates[J]. Endoscopy, 2010, 42(5): 389-394. DOI: 10.1055/s-0029-1243841 [14] SHAH T, ZFASS AM. Accuracy of EUS-FNA in solid pancreatic lesions: Sometimes size does matter[J]. Dig Dis Sci, 2019, 64(7): 1734-1735. DOI: 10.1007/s10620-019-5468-2 [15] ZHANG Y, ZHU Q, GONG TT, et al. Diagnostic value of EUS-FNA for pancreatic masses and its influential factors[J]. Chin J Dig Endosc, 2011, 28(9): 492-496. (in Chinese) https://www.cnki.com.cn/Article/CJFDTOTAL-YBQJ202006002.htm张燚, 诸琦, 龚婷婷, 等. 内镜超声引导下细针穿刺抽吸术对胰腺占位病变诊断价值及其影响因素的研究[J]. 中华消化内镜杂志, 2011, 28(9): 492-496. https://www.cnki.com.cn/Article/CJFDTOTAL-YBQJ202006002.htm [16] FAN Z, ZHANG L, ZHANG XF, et al. The diagnostic value of endoscopic ultrasonography guided fine needle aspiration for occupying pancreatic lesions[J]. Chin J Dig Endosc, 2016, 33(12): 847-850. (in Chinese) https://www.cnki.com.cn/Article/CJFDTOTAL-ZHXH200705001.htm范震, 张乐, 张筱凤, 等. 内镜超声引导下细针穿刺活检对胰腺占位性病变的诊断价值[J]. 中华消化内镜杂志, 2016, 33(12): 847-850. https://www.cnki.com.cn/Article/CJFDTOTAL-ZHXH200705001.htm [17] BERGERON JP, PERRY KD, HOUSER PM, et al. Endoscopic ultrasound-guided pancreatic fine-needle aspiration: Potential pitfalls in one institution's experience of 1212 procedures[J]. Cancer Cytopathol, 2015, 123(2): 98-107. [18] SIDDIQUI AA, KOWALSKI TE, SHAHID H, et al. False-positive EUS-guided FNA cytology for solid pancreatic lesions[J]. Gastrointest Endosc, 2011, 74(3): 535-540. [19] LI YQ, WANG W, SUN LQ, et al. Factors influencing the diagnostic positivity of endoscopic ultrasound guided fine needle aspiration for small pancreatic cancer and the occurrence of postoperative advent events[J]. Chin J Pancreatol, 2018, 18(4): 224-227. (in Chinese) http://med.wanfangdata.com.cn/Paper/Detail?id=PeriodicalPaper_yxbx201804003李玉琼, 王伟, 孙力祺, 等. 影响EUS-FNA的小胰腺癌诊断阳性率及术后并发症发生的相关因素[J]. 中华胰腺病杂志, 2018, 18(4): 224-227. http://med.wanfangdata.com.cn/Paper/Detail?id=PeriodicalPaper_yxbx201804003 [20] GLEESON FC, KIPP BR, CAUDILL JL, et al. False positive endoscopic ultrasound fine needle aspiration cytology: Incidence and risk factors[J]. Gut, 2010, 59(5): 586-593. [21] YOSHINAGA S, ITOI T, YAMAO K, et al. Safety and efficacy of endoscopic ultrasound-guided fine needle aspiration for pancreatic masses: A prospective multicenter study[J]. Dig Endosc, 2020, 32(1): 114-126. 期刊类型引用(9)
1. 马洁云,田晓锋,刘林霞,佟广海,陆博文,苏小琴,邰国梅. 肿瘤细胞Vimentin联合超声内镜引导细针穿刺活检术对胰腺实性肿瘤的诊断价值. 中国内镜杂志. 2024(11): 53-58 .  百度学术
百度学术2. 李振伟. 胰腺囊性病变诊断中多层螺旋CT的应用及临床意义分析. 实用医学影像杂志. 2023(03): 214-217 .  百度学术
百度学术3. 闫赵斌,李明彦,李振华,聂山文,饶鹏鹏. 超声内镜检查对胰腺癌及其临床分期的诊断价值. 癌症进展. 2023(15): 1684-1686+1701 .  百度学术
百度学术4. 卓宇果,高青. 超声内镜引导细针穿刺对胰腺占位病变诊断的影响因素. 胃肠病学和肝病学杂志. 2023(10): 1195-1198 .  百度学术
百度学术5. 于文昊,任利,王海久,王志鑫,孔繁玉,樊海宁. 超声内镜检查在胆胰壶腹疾病诊断中的应用. 中华消化外科杂志. 2023(12): 1414-1418 .  百度学术
百度学术6. 张朋飞,梁荔,张明,邢国强,牛帅,逄淑东,安伟. 三维重建联合超声内镜检查在胆道肿瘤术前精准评估中的应用价值. 中华消化外科杂志. 2023(12): 1490-1494 .  百度学术
百度学术7. 梁舟生,麦华卓,吕华亮,杨智娟,刘国连. 病毒性肺炎与其他感染性肺炎超声诊断价值对比研究. 影像研究与医学应用. 2022(03): 29-31 .  百度学术
百度学术8. 叶浩,易晓雷,李旭辉,汪杰,张军,刘志鹏,鲁柱. 宫颈鳞癌胰腺转移致梗阻性黄疸1例报告. 临床肝胆病杂志. 2022(03): 646-648 .  本站查看
本站查看9. 郭永吉,张丽艳,曲红梅,丁金秀,王秋野,李蕾,孙善明. 超声内镜引导下细针穿刺抽吸术对小胰腺癌诊断阳性率及术后并发症的影响. 中国内镜杂志. 2022(12): 63-67 .  百度学术
百度学术其他类型引用(2)
-




 PDF下载 ( 1985 KB)
PDF下载 ( 1985 KB)

 下载:
下载:


 下载:
下载:
 百度学术
百度学术