巨噬细胞对小鼠诱导多能干细胞向肝祖细胞分化的影响
DOI: 10.3969/j.issn.1001-5256.2021.04.025
利益冲突声明:本研究不存在研究者、伦理委员会成员、受试者监护人以及与公开研究成果有关的利益冲突。
作者贡献声明:宫甜甜、雷蕾、单智焱负责课题设计,资料分析,撰写论文;黄少刚、申景岭参与收集数据,修改论文;孙瑞珍、李秋明负责拟定写作思路,指导撰写文章并最后定稿。
Effect of macrophages on the differentiation of mouse induced pluripotent stem cells into hepatic progenitor cells
-
摘要:
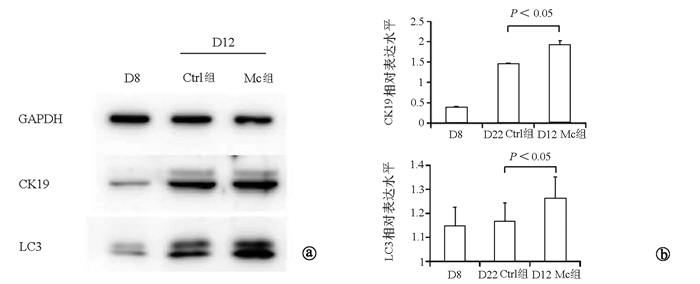
目的 探讨巨噬细胞(Mc)对小鼠诱导多能干细胞(iPSCs)向肝祖细胞(HPCs)分化的影响。 方法 C57BL/6N小鼠24只,采取腹腔冲洗法获得巨噬细胞,收集上清获得巨噬细胞条件培养基(Mc-CDM)。通过激活素A、骨形态发生蛋白4和成纤维细胞生长因子等诱导小鼠iPSCs向HPCs分化。将HPCs的诱导分为两组,一组使用正常培养基,为对照组(Ctrl组); 另一组在诱导D5使用Mc-CDM培养基,为实验组(Mc组)。通过形态学、免疫荧光、Western Blot检测方法,比较正常Ctrl组与Mc组HPCs的形态及相关蛋白表达的差异。计量资料两组间比较采用t检验。 结果 在体外建立了iPSCs来源的HPCs;HPCs具有向肝细胞分化的潜能。免疫荧光结果显示:与第12天的Ctrl组相比,第12天的Mc组的肝祖细胞特异性蛋白CK19的表达显著增高(0.901±0.072 vs 0.686±0.097,t=-3.093,P < 0.05);Western Blot结果显示:与第12天Ctrl组相比,第12天Mc组肝祖细胞相关蛋白CK19的表达显著增高(1.922±0.103 vs 1.448±0.012,t=-7.881,P < 0.05);同时,第12天Mc组自噬相关蛋白LC3的表达亦显著增高(1.392±0.042 vs 1.101±0.048,t=-5.978,P < 0.05)。 结论 巨噬细胞可促进小鼠iPSCs向HPCs分化,其机制可能与HPCs细胞自噬水平增加有关。 -
关键词:
- 多能干细胞 /
- 肝祖细胞 /
- 巨噬细胞 /
- 细胞分化 /
- 小鼠, 近交C57BL
Abstract:Objective To investigate the effect of macrophages (MCs) on the differentiation of mouse induced pluripotent stem cells (iPSCs) into hepatic progenitor cells (HPCs). Methods A total of 24 C57BL/6N mice were used to obtain MCs by peritoneal irrigation, and the supernatant was collected to obtain the conditioned medium of MCs (MC-CDM). Activin A, bone morphogenetic protein 4, and fibroblast growth factor were used to induce the differentiation of mouse iPSCs into HPCs. The differentiation of HPCs were randomly divided into control group (normal medium) and experimental group (MC group; use of MC-CDM medium on day 5 of induction). Morphology, immunofluorescence assay, and Western blot were used to compare the morphology of HPCs and the expression of related proteins between the control group and the MC group. The t-test was used for comparison of continuous data between two groups. Results HPCs derived from iPSCs were established in vitro, and HPCs had the potential to differentiate into hepatocytes. Immunofluorescence assay showed that compared with the D12 control group, the D12 MC group had a significant increase in the protein expression of the HPC-specific protein CK19 (0.901±0.072 vs 0.686±0.097, t=-3.093, P < 0.05). Western blot showed that compared with the D12 control group, the D12 MC group had a significant increase in the protein expression of the HPC-related protein CK19 (1.922±0.103 vs 1.448±0.012, t =-7.881, P < 0.05), as well as a significant increase in the protein expression of the autophagy-related protein LC3 (1.392±0.042 vs 1.101±0.048, t =-5.978, P < 0.05). Conclusion MCs can promote the differentiation of mouse iPSCs into HPCs, possibly by increasing the autophagy level of HPCs. -
-
[1] KATOONIZADEH A, POUSTCHI H. Adult hepatic progenitor cell niche: How it affects the progenitor cell fate[J]. Middle East J Dig Dis, 2014, 6(2): 57-64. http://pubmedcentralcanada.ca/pmcc/articles/PMC4034666/ [2] LIU LP, YANNAM GR, NISHIKAWAT, et al. The microenvironment in hepatocyte regeneration and function in rats with advanced cirrhosis[J]. Hepatology, 2012, 55: 1529-1539. DOI: 10.1002/hep.24815 [3] SALATI S, LISIGNOLI G, MANFERDINI C, et al. Co-culture of hematopoietic stem/progenitor cells with human osteblasts favours mono/macrophage differentiation at the expense of the erythroid lineage[J]. PLoS One, 2013, 8(1): e53496. DOI: 10.1371/journal.pone.0053496 [4] ZHAO SJ, KONG FQ, JIE J, et al. Macrophage MSR1 promotes BMSC osteogenic differentiation and M2-like polarization by activating PI3K/AKT/GSK3β/β-catenin pathway[J]. Theranostics, 2020, 10(1): 17-35. DOI: 10.7150/thno.36930 [5] ZHOU QJ, XIANG LX, SHAO JZ, et al. In vitro differentiation of hepatic progenitor cells from mouse embryonic stem cells induced by sodium butyrate[J]. J Cell Biochem, 2007, 100(1): 29-42. DOI: 10.1002/jcb.20970 [6] YANAGIDA A, NAKAUCHI H, KAMIYA A. Generation and in vitro expansion of hepatic progenitor cells from human iPS cells[J]. Methods Mol Biol, 2016, 1357: 295-310. [7] BELLANTI F, PANNONE G, TARTAGLIA N. Redox control of the immune response in the hepatic progenitor cell niche[J]. Front Cell Dev Biol, 2020, 8: 295. DOI: 10.3389/fcell.2020.00295 [8] CHEN Y, WANG B, ZHOU H, et al. Autophagy is required for the maintenance of liver progenitor cell functionality[J]. Cell Physiol Biochem, 2015, 36(3): 1163-1174. DOI: 10.1159/000430287 [9] CHANG NC. Autophagy and stem cells: Self-eating for self-renewal[J]. Front Cell Dev Biol, 2020, 8: 138. DOI: 10.3389/fcell.2020.00138 [10] CHEN XD, TAN JL, FENG Y, et al. Autophagy in fate determination of mesenchymal stem cells and bone remodeling[J]. World J Stem Cells, 2020, 12(8): 776-786. DOI: 10.4252/wjsc.v12.i8.776 [11] TOMCZYK S, SUKNOVIC N, SCHENKELAARS Q, et al. Deficient autophagy in epithelial stem cells drives aging in the freshwater cnidarian Hydra[J]. Development, 2020, 147(2): dev177840. DOI: 10.1242/dev.177840 期刊类型引用(9)
1. 刘韦,白浪. 慢加急性肝衰竭动物模型研究现状. 临床肝胆病杂志. 2024(01): 187-192 .  本站查看
本站查看2. 陈然,王帅,高扬,杨志琴,徐严. HBV相关慢加急性肝衰竭合并脓毒症的诊疗进展. 肝脏. 2024(07): 867-870 .  百度学术
百度学术3. 刘扬,洪文,黄克林,杨博,刘亚坡,路明. 粪菌移植对顽固性便秘小鼠的肠道菌群和肠道动力以及TLR4/NF-κB通路蛋白的影响. 现代生物医学进展. 2024(16): 3020-3024 .  百度学术
百度学术4. 于丹丹,刘亚坡,洪文,杨博,张媛. 粪菌移植对顽固性便秘患者肠道功能、免疫功能及炎症反应的改善效果. 结直肠肛门外科. 2024(05): 578-583 .  百度学术
百度学术5. 阮浩龙,王宁,于鸿浩,岳鹏鹏. 基于基因编辑技术研究特定基因对小鼠肠道微生物的影响. 中国医学工程. 2024(11): 44-49 .  百度学术
百度学术6. 徐洪凯,汪春付,张野,连建奇. 粪菌移植在慢性肝病治疗中的应用. 临床肝胆病杂志. 2023(09): 2237-2243 .  本站查看
本站查看7. 周荃,蔡春琳,李金强. 肠-肝轴:肠道微生物稳态与肝细胞癌. 临床肝胆病杂志. 2023(11): 2710-2717 .  本站查看
本站查看8. 周宜,刘晓琴,吴学敏,张浩,王海琴,王红亮,孟祥龙. 乳香及其炮制品对葡聚糖硫酸钠诱导小鼠炎症性肠病的保护作用. 现代药物与临床. 2022(07): 1432-1438 .  百度学术
百度学术9. 刘蕾,高越颖,郭琳,邱立朋,李会,耿燕. 酒精性肝损伤保护的药理实验教学设计. 实验室研究与探索. 2022(05): 238-243 .  百度学术
百度学术其他类型引用(2)
-




 PDF下载 ( 3026 KB)
PDF下载 ( 3026 KB)


 下载:
下载:


 百度学术
百度学术

