肝硬化重度食管胃静脉曲张治疗后再出血及5年预后影响因素分析
DOI: 10.3969/j.issn.1001-5256.2021.06.022
Rebleeding and prognosis of patients with liver cirrhosis and severe esophagogastric variceal bleeding
-
摘要:
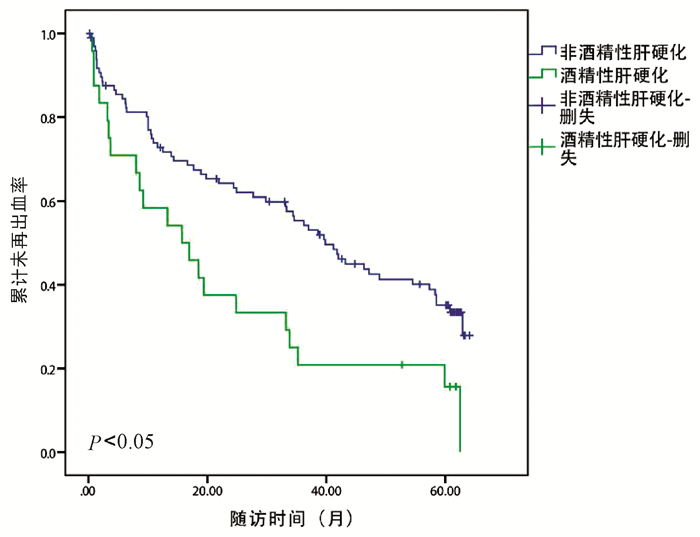
目的 探讨肝硬化并发重度食管胃静脉曲张破裂出血(EVB)患者5年内再出血的危险因素及影响其5年生存的相关因素。 方法 回顾性选取2012年5月—2014年5月因重度首次EVB于天津市第三中心医院就诊的肝硬化129例患者,随访时间为5年。分析患者年龄、性别、肝硬化原因、首次出血时是否合并感染、肝硬度(LSM)、脾硬度(SSM)、门静脉内径、生化学指标、再出血时间及预后等临床资料。以食管胃静脉曲张再出血为主要研究终点,以死亡为次要研究终点。计量资料2组间比较采用t检验;计数资料2组间比较采用χ2检验;分别采用logistic回归分析法和Cox回归分析法筛选出EVB患者治疗后再次出血的独立危险因素及5年的生存预测指标,Kaplan-Meier曲线分析累积未再出血率。 结果 129例患者中有87例(67.4%)患者在随访期间发生再出血。再出血组与未再出血组患者比较,酒精性肝硬化患者比例(χ2=4.896,P=0.027)、门静脉内径(t=2.203,P=0.030)、LSM(Z=-2.771,P=0.006)和SSM(t=2.678,P=0.010)差异均有统计学意义。酒精性肝硬化患者的平均出血次数和累计出血率显著高于非酒精性肝硬化患者(P值均<0.05)。多因素logistic回归分析显示酒精性肝硬化、LSM和SSM是EVB患者治疗后5年内再出血的独立危险因素(OR值分别为5.687、1.039、1.078,95%CI分别为1.230~26.129、1.010~1.070、1.028~1.129,P值分别为0.025、0.007、0.001)。129例患者中死亡45例(34.9%)。Cox单因素分析显示,死亡组与存活组在年龄、出血次数、平均动脉压、门静脉内径、AST、淋巴细胞百分比以及首次出血是否感染等均存在明显差异(P值均<0.05)。进一步多因素分析表明,患者5年生存率与门静脉内径、年龄、出血次数以及首次出血时是否感染有关(OR值分别为1.459、1.053、1.286、5.239,95%CI分别为1.056~2.014、1.006~1.103、1.040~1.591、1.750~15.641,P值分别为0.022、0.026、0.020、0.003)。 结论 酒精性肝硬化、LSM和SSM是影响EVB患者5年内发生再出血的独立危险因素,而年龄、出血次数、门静脉内径以及首次出血时是否合并感染与5年预后有关。 Abstract:Objective To investigate the risk factors for rebleeding within 5 years and the influencing factors for 5-year survival in patients with liver cirrhosis and severe esophagogastric variceal bleeding (EVB). Methods A retrospective analysis was performed for 129 patients with liver cirrhosis who attended Tianjin Third Central Hospital from May 2012 to May 2014 due to severe EVB for the first time, with a follow-up time of 5 years. Related clinical data were analyzed, including age, sex, cause of liver cirrhosis, presence or absence of infection at the first time of bleeding, liver stiffness measurement (LSM), splenic stiffness measurement (SSM), portal vein diameter, biochemical parameters, rebleeding time, and prognosis. Esophagogastric variceal rebleeding was defined as the primary endpoint and death was defined as the secondary endpoint. The t-test was used for comparison of continuous data between two groups, and the chi-square test was used for comparison of categorical data between two groups; a logistic regression analysis was used to investigate the independent risk factors for rebleeding, and a Cox regression analysis was used to analyze the predictive indicators for 5-year survival in EVB patients; the Kaplan-Meier curve was used to analyze the cumulative non-rebleeding rate. Results Among the 129 patients, 87(67.4%) experienced rebleeding during follow-up. There were significant differences between the rebleeding group and the non-rebleeding group in the proportion of patients with alcoholic cirrhosis (χ2=4.896, P=0.027), portal vein diameter (t=2.203, P=0.030), LSM(Z=-2.771, P=0.006), and SSM(t=2.678, P=0.010). The patients with alcoholic cirrhosis had a significantly higher mean number of times of bleeding than those with non-alcoholic cirrhosis (all P < 0.05). The multivariate logistic regression analysis showed that alcoholic cirrhosis (odds ratio [OR]=5.687, 95% confidence interval [CI]: 1.230-26.129, P=0.025), LSM(OR=1.039, 95% CI: 1.010-1.070, P=0.007), and SSM(OR=1.078, 95% CI: 1.028-1.129, P=0.001) were independent risk factors for rebleeding within 5 years after treatment in EVB patients. Among the 129 patients, 45 (34.9%) died. The univariate Cox regression analysis showed that there were significant differences between the death group and the survival group in age, times of bleeding, mean arterial pressure, portal vein diameter, aspartate aminotransferase, lymphocyte percentage, and presence or absence of infection at the first time of bleeding (all P < 0.05). Further multivariate analysis showed that 5-year survival rate was associated with portal vein diameter (OR=1.459, 95% CI: 1.056-2.014, P=0.022), age (OR=1.053, 95% CI: 1.006-1.103, P=0.026), times of bleeding (OR=1.286, 95% CI: 1.040-1.591, P=0.020), and presence or absence of infection at the first time of bleeding (OR=5.239, 95% CI: 1.750-15.641, P=0.003). Conclusion Alcoholic cirrhosis, LSM, and SSM are independent risk factors for rebleeding within 5 years in EVB patients, and age, times of bleeding, portal vein diameter, and presence or absence of infection at the first time of bleeding are associated with 5-year survival. -
Key words:
- Liver Cirrhosis /
- Esophageal And Gastric Varices /
- Hemorrhage /
- Risk Factors
-
肝细胞癌(hepatocellular carcinoma, HCC)是全球第六大常见肿瘤和我国第二大癌症死亡原因,早期发现、及时采取有效的治疗是改善患者生存预后的重要手段[1-2]。射频消融术(radiofrequency ablation,RFA)在早期肝癌特别是直径≤2 cm的患者中的疗效与手术切除相当,是不能切除的早期肝癌患者的首选治疗方法[3-6]。许多临床和实验研究[7-8]表明,全身炎症反应在肿瘤的进展中发挥重要作用。近年来,多项研究[9-11]证实了全身炎症标志物包括中性粒细胞/淋巴细胞比值(neutrophil-to-lymphocyte ratio, NLR)、红细胞分布宽度/淋巴细胞比值(red blood cell distribution width-to-lymphocyte ratio, RLR)、淋巴细胞/单核细胞比值(lymphocyte-to- monocyte ratio, LMR)等在包括HCC在内的恶性肿瘤和肝衰竭预后中的作用。本研究旨在探讨术前全身炎症标志物NLR、RLR及LMR在早期小肝癌RFA后预后中的价值。
1. 资料与方法
1.1 研究对象
选取2011年9月—2020年12月在天津市第二人民医院行RFA的132例初诊HCC患者。HCC的诊断及分期符合《原发性肝癌诊疗规范(2019年版)》[2],基于动态增强MRI/CT、超声造影等2种或以上影像学支持,具体表现为动脉期病灶明显强化,门静脉期和/或平衡期病灶强化低于肝实质即“快进快出”的肝癌典型特征,伴或不伴AFP水平升高。纳入标准:(1)单个病灶直径≤3 cm;(2)巴塞罗那临床(BCLC)分期为0/A期;(3)不适合手术切除或者患者拒绝手术切除治疗;(4)接受RFA作为首次治疗。排除标准:(1)Child-Pugh分级为C级;(2)合并其他恶性肿瘤或严重心脑血管病等;(3)2周内服用过抗炎药物;(4)随访资料不全或围术期死亡;(5)随访时间<3个月。
1.2 研究方法
收集RFA前1周内的数据。一般资料:性别、年龄、BMI;病因学资料:HBV、HCV、代谢相关脂肪性肝病(MAFLD)和乙醇摄入等;实验室指标:血常规、肝功能、肾功能、AFP等;影像学资料:CT、磁共振成像(MRI)、腹部彩超、超声造影等。
1.3 RFA方案
在超声引导下经皮射频消融。严格按照《2011 CSLC/CSCO/CMA肝癌射频消融治疗规范的专家共识》流程进行。患者在穿刺部位接受2%盐酸利多卡因治疗,并静脉滴注50 mg盐酸哌替啶与50 mL 5%葡萄糖水混合。手术过程中持续监测心血管和呼吸系统。对于所有肿瘤,消融边缘至少应达到肿瘤边缘以外5 mm。完全消融定义为肝脏动态增强MRI/CT或超声造影提示肿瘤所在区域为低密度(超声表现为高回声),动脉期无强化。术后28 d复查动态增强MRI/CT及动脉造影评估肿瘤是否完全消融。对于未完全消融者再次消融,直至肿瘤完全反应。
1.4 患者随访
本研究的目标事件为无复发生存(recurrence free survival, RFS)和总生存(overall survival,OS)。RFS时间定义为从RFA术后到首次复发或死亡之间的时间。OS定义为从RFA术后到患者死亡或随访截止日期之间的时间。完全消融后,患者前2年内,每3个月复查1次AFP及动态增强MRI/CT、超声造影等,之后每半年监测1次上述指标,如动态增强MRI/CT和/或超声造影提示原病灶周围或者肝内远处出现动脉期异常强化影,门脉期及平衡期密度降低,伴或不伴AFP升高,考虑肿瘤复发,记录复发肿瘤的位置、数目、大小、有无血管侵犯等。通过多学科会诊综合评估患者情况,给予针对肝癌复发的相关治疗。治疗后继续随访,直至患者死亡或研究截止日期2021年6月30日。
1.5 统计学方法
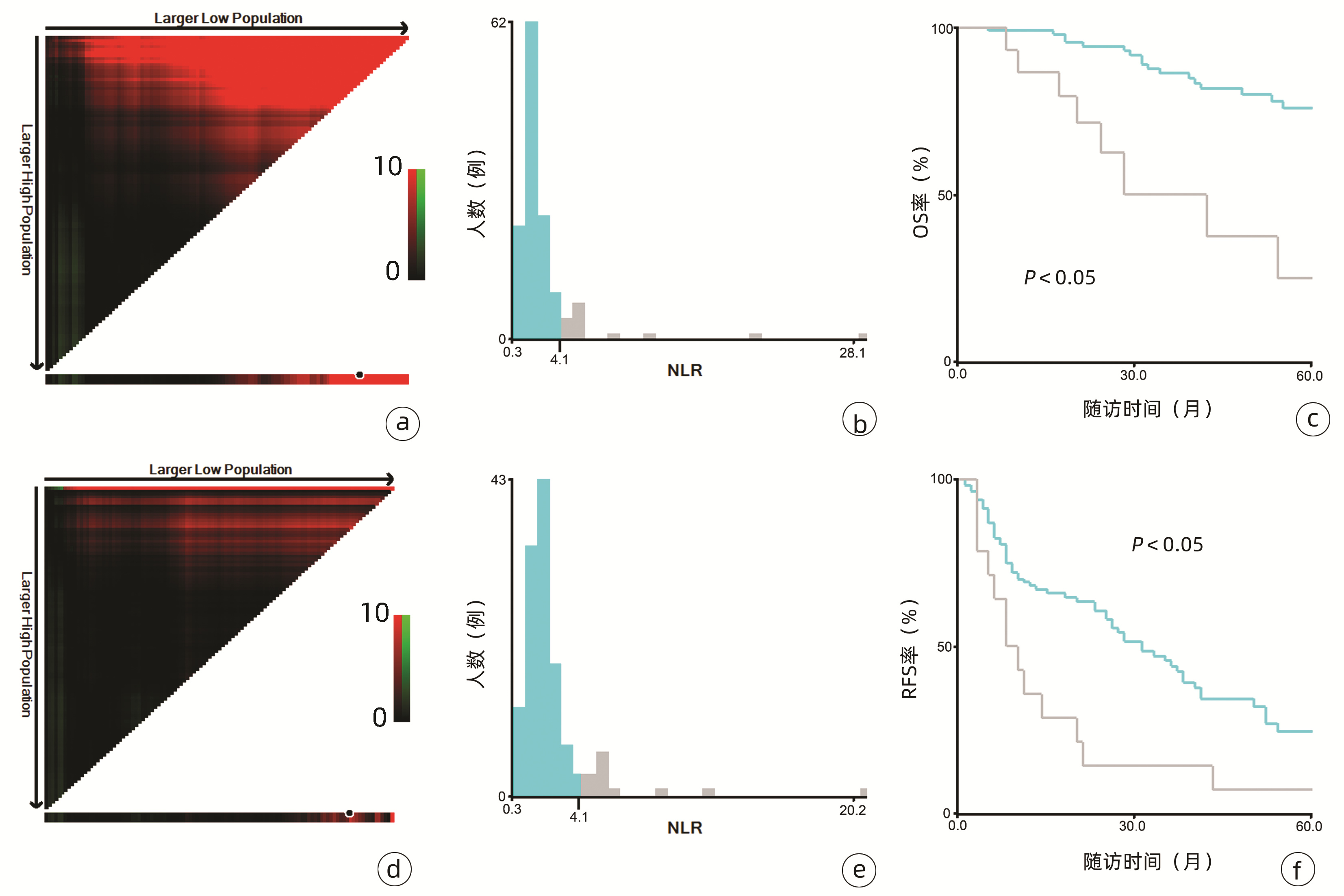
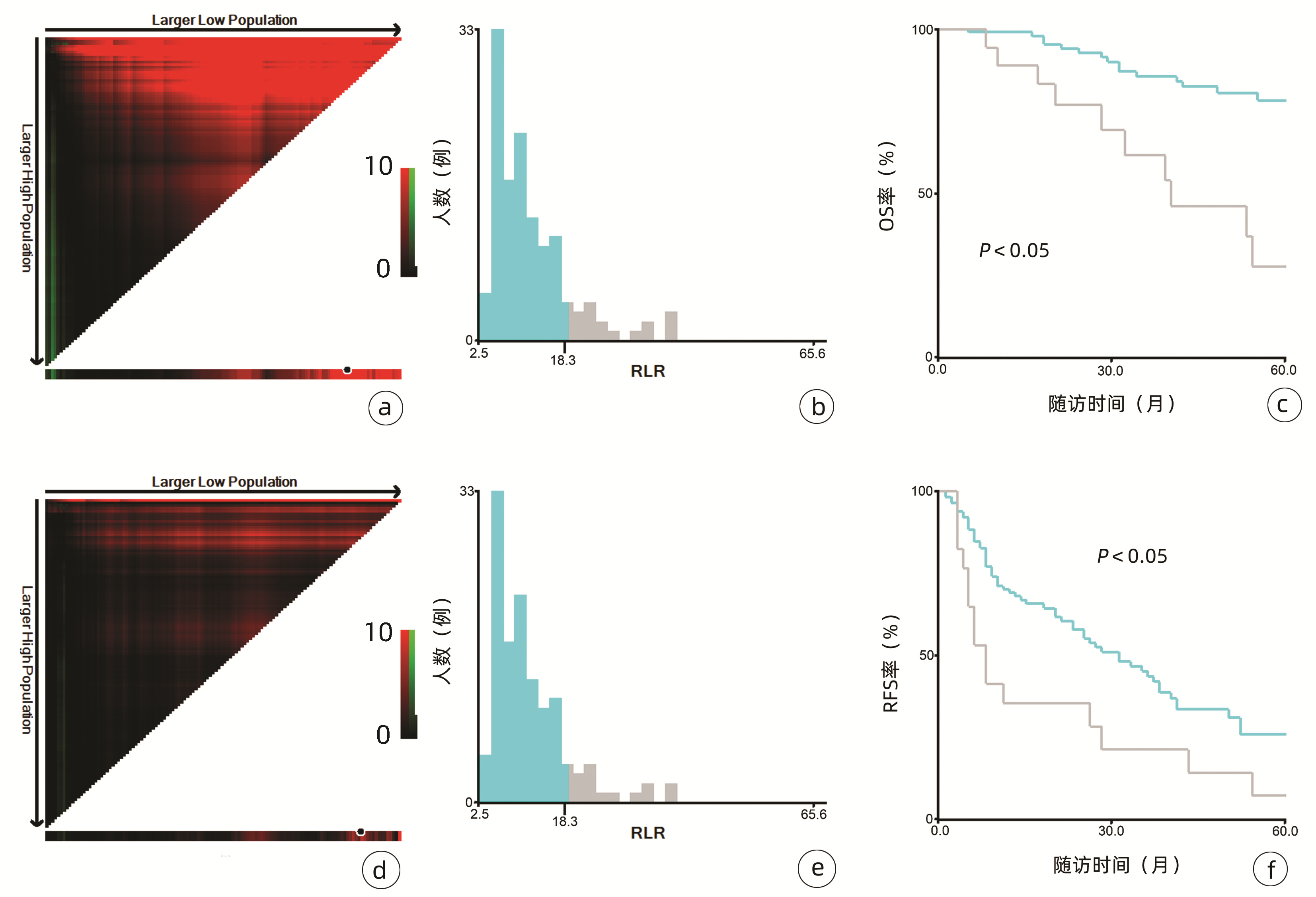
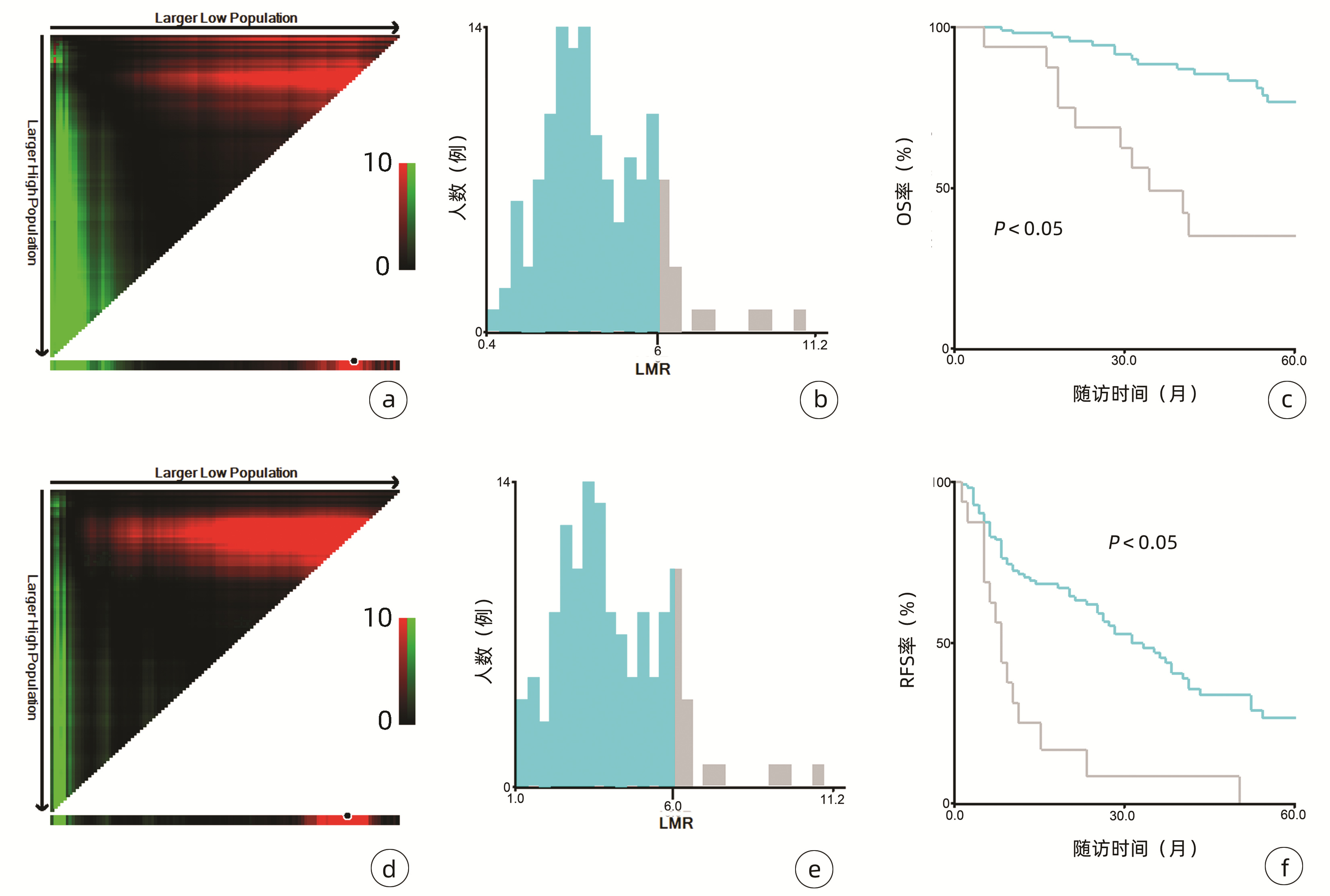
采用SPSS 22.0软件及R语言4.0.3进行统计分析。计数资料组间比较采用χ2检验。运用X-tile工具以5年生存率及RFS率为结局事件,采用“枚举法”确定NLR、RLR和LMR的最佳截断值[12]。使用Kaplan-Meier方法绘制生存曲线,并采用log-rank检验比较复发及生存的组间差异。单因素分析采用log-rank检验,将log-rank检验分析中具有统计学意义的因素纳入多因素Cox回归分析以确定RFS率与OS率的危险因素,计算风险比(HR)及其95%CI。P<0.05为差异有统计学意义。
2. 结果
2.1 一般资料
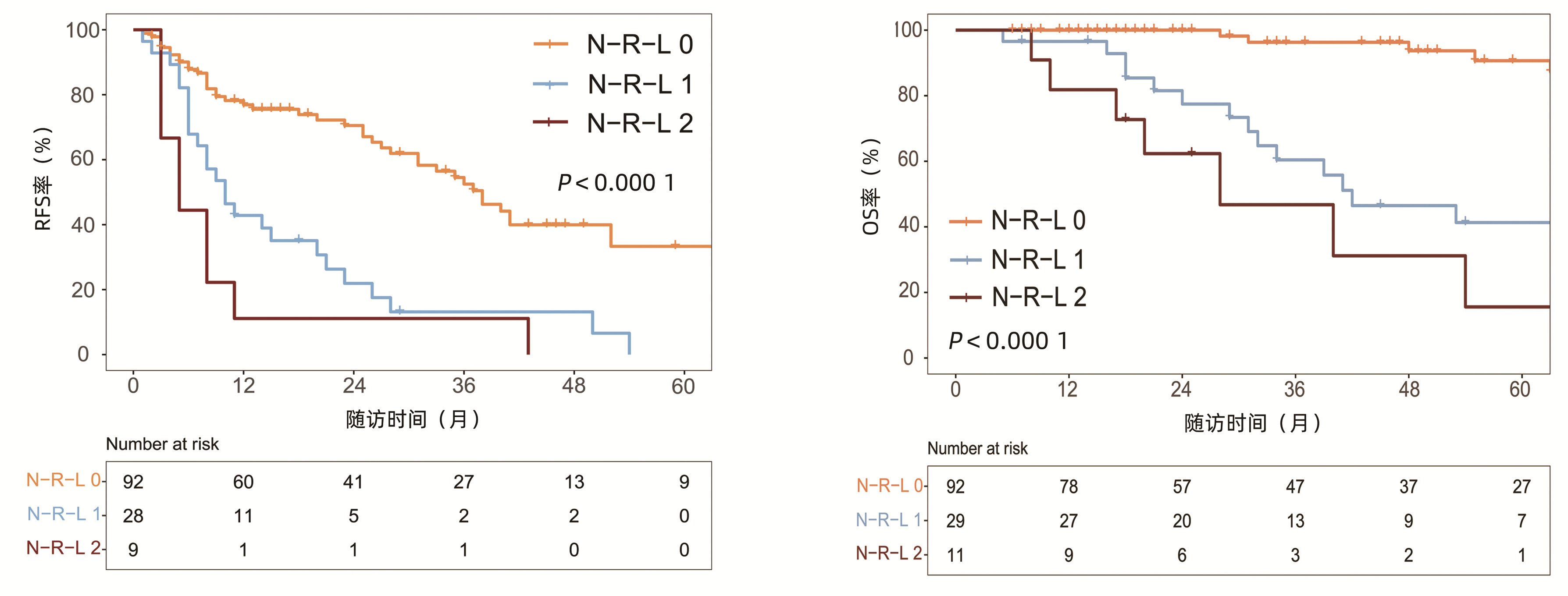
132例患者中男性96例,平均年龄(58.50±9.66)岁。利用患者数据对5年OS和RFS进行X-tile分析,确定NLR、RLR和LMR的最佳截断值分别为4.1、18.3和6(图 1~3)。根据NLR、RLR、LMR分组与OS及RFS的相关性,将NLR、RLR及LMR按积分计算(表 1),根据N-R-L评分的得分情况将患者分为3组:N-R-L 0分组,N-R-L 1分组和N-R-L 2分组,并将其作为OS和RFS的预后指标。3组患者Child-Pugh分级、Alb比较差异均有统计学意义(P值均<0.05)(表 2)。
表 1 N-R-L评分的计分及分组方式Table 1. Scoring and grouping of N-R-L score变量 得分 NLR <4.1 0 ≥4.1 1 RLR <18.3 0 ≥18.3 1 LMR <6 0 ≥6 1 N-R-L NLR+RLR+LMR=0 0 NLR+RLR+LMR=1 1 NLR+RLR+LMR=2 2 注:N-R-L,NLR、RLR和LMR三项评分之和。 表 2 术前N-R-L分组患者的临床特征Table 2. Clinical characteristics of patients in preoperative N-R-L group临床特征 N-R-L 0分组(n=92) N-R-L 1分组(n=29) N-R-L 2分组(n=11) χ2值 P值 性别[例(%)] 0.545 0.762 男 66(71.7) 21(72.4) 9(81.8) 女 26(28.3) 8(27.6) 2(18.2) 年龄[例(%)] 0.371 0.831 <60岁 48(52.2) 17(58.6) 6(54.5) ≥ 60岁 44(47.8) 12(41.4) 5(45.5) BMI[例(%)] 2.024 0.363 <24 kg/m2 42(45.7) 9(31.0) 5(45.5) ≥24 kg/m2 50(54.3) 20(69.0) 6(54.5) Child-Pugh分级[例(%)] 10.992 0.001 A级 81(88.0) 26(89.7) 4(36.4) B级 11(12.0) 3(10.3) 7(63.6) BCLC分期[例(%)] 3.640 0.056 0期 18(19.6) 3(10.3) 0 A期 74(80.4) 26(89.7) 11(100) 肿瘤直径[例(%)] 3.876 0.144 <2 cm 53(57.6) 17(58.6) 3(27.3) ≥2 cm 39(42.4) 12(41.4) 8(72.7) HBV感染[例(%)] 1.601 0.449 否 22(23.9) 5(17.2) 4(36.4) 是 70(76.1) 24(82.8) 7(63.6) HCV感染[例(%)] 1.029 0.598 否 76(82.6) 22(75.9) 8(71.7) 是 16(17.4) 7(24.1) 3(27.3) MAFLD[例(%)] 0.367 0.832 否 56(60.9) 16(55.2) 7(63.6) 是 36(39.1) 13(44.8) 4(36.4) AFP[例(%)] 1.266 0.531 <15 ng/mL 59(64.1) 21(72.4) 6(54.5) ≥15 ng/mL 33(35.9) 8(27.6) 5(45.5) ALBI分级[例(%)] 2.845 0.092 1级 54(58.7) 16(55.2) 5(45.4) 2级 36(39.1) 13(44.8) 3(27.3) 3级 2(2.2) 0 3(27.3) TBil[例(%)] 0.904 0.636 ≤20 μmol/L 55(59.8) 16(55.2) 5(45.5) >20 μmol/L 37(40.2) 13(44.8) 6(54.5) Alb[例(%)] 5.699 0.017 ≥35 g/L 82(89.1) 23(79.3) 7(63.6) <35 g/L 10(10.9) 6(20.7) 4(36.4) ALT[例(%)] 0.109 0.741 ≤50 U/L 76(82.6) 28(96.6) 7(63.6) >50 U/L 16(17.4) 1(3.4) 4(36.4) AST[例(%)] 1.572 0.456 ≤40 U/L 71(77.2) 24(82.8) 7(63.6) >40 U/L 21(22.8) 5(17.2) 4(36.4) MELD评分[例(%)] 3.022 0.221 <9分 65(70.7) 18(62.1) 5(45.5) ≥9分 27(29.3) 11(37.9) 6(54.5) 注:ALBI分级,白蛋白-胆红素分级。 2.2 RFA术后的复发和生存
中位随访时间为34个月(5~111个月)。所有患者的1、3和5年RFS率分别为64.3%、40.0%和21.5%,中位RFS为26个月;1、3和5年OS率分别为97.6%、82.1%和69.8%。N-R-L 0分组、N-R-L 1分组和N-R-L 2分组的1、3和5年RFS率分别为76.9%、52.5%、33.3%,42.9%、13.1%、0和11.1%、0、0(χ2=35.345,P<0.000 1)(图 4);3组的1、3和5年OS率分别为100%、96.3%、90.7%,96.6%、60.4%、41.3%和81.8%、46.8%、15.6%(χ2=38.460,P<0.000 1)(图 4)。N-R-L 0分组、N-R-L 1分组和N-R-L 2分组的中位RFS分别为38个月、10个月和5个月,组间差异有统计学意义(χ2=35.345,P<0.000 1)。
2.3 RFS率的预测因素
对患者RFA术前相关指标进行单因素及多因素分析,以确定RFS率的预测因素(表 3)。log-rank检验结果显示,男性、Child-Pugh B级、肿瘤直径≥2 cm、TBil>20 μmol/L、Alb>35 g/L、ALBI分级2/3级以及N-R-L评分1/2分与RFS率显著相关(P值均<0.05)。进一步行多因素Cox回归分析结果表明,肿瘤直径≥2 cm(HR=2.10, 95%CI: 1.28~3.43,P=0.003)、N-R-L评分1分(HR= 3.14, 95%CI: 1.81~5.46,P<0.000 1)以及N-R-L评分2分(HR=2.61, 95%CI: 1.06~6.42,P=0.037)是RFS率的独立预测因素(P值均<0.05)。
表 3 Cox回归分析RFS率的影响因素Table 3. Results of RFS by Cox regression analysis变量 单因素分析 多因素分析 HR(95%CI) P值 HR(95%CI) P值 性别(男/女) 1.97(1.14~3.40) 0.015 1.55(0.87~2.76) 0.136 BMI(≥24 kg/m2/<24 kg/m2) 1.22(0.77~1.92) 0.402 MAFLD(是/否) 1.03(0.65~1.61) 0.916 Child-Pugh分级(B级/A级) 2.58(1.50~4.45) 0.001 1.28(0.56~2.91) 0.564 肿瘤直径(≥2 cm/<2 cm) 2.28(1.43~3.62) 0.001 2.10(1.28~3.43) 0.003 BCLC分期(A期/0期) 2.06(0.89~4.75) 0.090 ALT(>50 U/L/≤50 U/L) 1.41(0.78~2.57) 0.260 AST(>40 U/L/≤40 U/L) 1.63(0.98~2.70) 0.058 TBil(≤20 μmol/L/>20 μmol/L) 1.69(1.07~2.65) 0.024 1.60(0.96~2.67) 0.074 Alb(<35 g/L/≥35 g/L) 2.45(1.45~4.12) 0.001 1.01(0.44~2.32) 0.974 AFP(>400 ng/mL/≤400 ng/mL) 0.81(0.33~2.01) 0.650 ALBI分级(ref: 1级) 2级 1.25(0.79~2.00) 0.341 0.92(0.49~1.70) 0.781 3级 4.15(1.61~10.70) 0.003 1.91(0.48~7.59) 0.356 MELD评分(>9分/≤9分) 1.44(0.91~2.28) 0.124 N-R-L得分(ref: 0分) 1分 3.13(1.89~5.20) <0.000 1 3.14(1.81~5.46) <0.000 1 2分 4.99(2.40~10.37) <0.000 1 2.61(1.06~6.42) 0.037 2.4 OS率的预测因素
表 4总结了RFA术后OS率的预测因素。log-rank检验结果提示Child-Pugh B级、肿瘤直径≥2 cm、TBil>20 μmol/L、Alb>35 g/L、ALBI分级2/3级以及N-R-L评分1/2分与OS显著相关。多因素Cox回归结果表明,肿瘤直径≥2 cm(HR=3.67, 95%CI: 1.58 ~ 8.52,P=0.002)、N-R-L评分1分(HR=8.27, 95%CI: 3.15 ~ 21.71,P<0.000 1)以及N-R-L评分2分(HR=14.59, 95%CI: 3.96 ~ 53.78,P<0.000 1)是OS率的独立预测因素。
表 4 Cox回归分析OS率的影响因素Table 4. Results of OS by Cox regression analysis变量 单因素分析 多因素分析 HR(95%CI) P值 HR(95%CI) P值 性别(男/女) 2.17(0.88~5.33) 0.093 BMI(≥24 kg/m2/<24 kg/m2) 1.64(0.78~3.44) 0.191 MAFLD(是/否) 1.20(0.58~2.51) 0.620 Child-Pugh分级(B级/A级) 3.54(1.52~8.25) 0.003 0.99(0.28~3.52) 0.987 肿瘤直径(≥2 cm/<2 cm) 2.89(1.36~6.14) 0.006 3.67(1.58~8.52) 0.002 BCLC分期(A期/0期) 1.10(0.33~3.63) 0.880 ALT(>50 U/L/≤50 U/L) 1.21(0.46~3.17) 0.705 AST(>40 U/L/≤40 U/L) 1.30(0.58~2.94) 0.530 TBil(≤20 μmol/L/>20 μmol/L) 2.27(1.08~4.76) 0.030 1.97(0.82~4.77) 0.132 Alb(<35 g/L/≥35 g/L) 3.49(1.58~7.74) 0.002 1.36(0.36~5.09) 0.649 AFP(>400 ng/mL/≤400 ng/mL) 1.35(0.41~4.50) 0.621 ALBI分级(ref: 1级) 2级 1.67(0.78~3.56) 0.184 0.97(0.35~2.65) 0.950 3级 12.94(2.55~65.68) 0.002 2.11(0.25~17.94) 0.495 MELD评分(>9分/≤9分) 1.59(0.75~3.33) 0.224 N-R-L得分(ref: 0分) 1分 6.92(2.82~17.00) <0.000 1 8.27(3.15~21.71) <0.000 1 2分 13.50(4.68~38.91) <0.000 1 14.59(3.96~53.78) <0.000 1 3. 讨论
多项研究表明,对于不可切除的早期小肝癌,RFA能提供良好的治疗效果。本研究选取了单发直径≤3 cm的早期/极早期HCC患者,将RFA作为首次治疗方法。在整个随访期内,共有77例(58.3%)复发,29例(22%)死亡,与先前研究[4, 13-14]结果类似。所有患者接受RFA治疗后,仅3例患者出现轻微并发症,经治疗后均好转,没有患者出现严重并发症,表明RFA安全性较高,更具成本效益。
评估了RFA率与OS率的危险因素,发现肿瘤直径≥2 cm、N-R-L评分1分及2分与患者不良预后独立相关。HCC的复发及预后的差异与初始肿瘤大小相关已经在先前的研究[15-16]中被证实。在HCC的发生发展中,随着病灶直径的增加,微浸润的发生率明显增加,这可能是HCC治疗后复发的重要原因。研究[4]表明,对于直径≤3 cm的HCC患者,HCC≥2 cm的微转移和微血管浸润更明显。另外,肿瘤直径增大可能造成消融不彻底,残存的微病灶直接导致复发,这些结果均会影响患者的生存预后。
炎症在肿瘤发生发展的各个阶段均发挥重要作用,可驱动肿瘤的发生、生长、进展和转移等重要过程[17-18]。除了局部肿瘤微环境外,全身性炎症反应通过干扰组织内环境稳态从而影响肿瘤的发展,特别是肿瘤的转移[19]。血常规作为反映全身炎症反应最普遍的工具,在临床广泛应用且易于获取。单独的血细胞计数可能与整体预后没有显著关系,但由这些细胞计数组合产生的全身炎症标志物如NLR、RLR和LMR等被认为是各类癌症的独立预测因素。
淋巴细胞是机体免疫监视和免疫应答的重要组成成分,参与肿瘤的免疫反应,特别是在抗肿瘤增殖和转移的过程[20]。淋巴细胞浸润增加与癌症患者预后良好相关[21],而淋巴细胞数量减少和功能抑制可能会削弱肿瘤特异性免疫,造成利于肿瘤侵袭和转移的环境,进而影响患者预后[22-23]。中性粒细胞可直接释放炎症因子,促进肿瘤血管生成,加速肿瘤的生长和转移[24]。NLR是反映中性粒细胞促肿瘤特性与淋巴细胞抗肿瘤宿主免疫反应之间平衡的一个因素,但在炎症环境下,随着中性粒细胞增加和淋巴细胞减少,这一平衡被打破,直接导致机体抗肿瘤能力下降。这提示机体处于抗肿瘤免疫抑制状态,炎症反应将向促肿瘤进展方向发展,最终导致患者预后不良。另外,炎症可以刺激单核细胞到外周血,进而被招募到肿瘤组织并分化为肿瘤相关巨噬细胞,进一步与肿瘤细胞相互作用促进肿瘤进展[25-26]。红细胞分布宽度(red blood cell distribution width, RDW)反映了红细胞在循环中体积分布的变化。有研究[27]表明RDW升高与HCC患者无病生存率较低和OS率较差相关。而作为RDW与淋巴细胞比值, RLR升高已被证明能够预测肝功能衰竭和结肠癌的不良预后,并与肝硬化的严重程度相关[9-10, 28]。
与先前结果一致,本研究亦明确了NLR、RLR和LMR在早期小肝癌RFA后的预后价值。由于先前研究对于上述炎症标志物的分组尚无统一标准,本研究采用X-tile工具基于患者5年OS及RFS计算出3个指标的最佳截断值分别为4.1、18.3和6,据此分组后三者在患者RFS及OS方面均表现出优异的预后价值,特别是将三者结合后得到的N-R-L评分,在N-R-L评分≥1与早期HCC首次RFA后的早期复发和预后不良显著相关,因此可作为早期HCC患者RFA治疗预后不良的独立预后生物标志物。
另外,本研究的大多数患者为HBV或HCV相关HCC,机体本身存在慢性持续性炎症。肿瘤相关炎症反应刺激炎症介质的释放,加之血细胞计数异常所致的抗肿瘤免疫功能下降,为肿瘤的侵袭和转移提供有利条件。提示炎症反应越明显,N-R-L评分越高,患者预后也越差。
总之,全身炎症反应标志物N-R-L评分作为一种简单、易于获取且成本极低的指标,有望成为有用的无创生物标志物,用以预测早期HCC患者RFA术后的复发和生存。
-
表 1 患者一般资料比较
指标 再出血组(n=87) 未再出血组(n=42) 统计值 P值 死亡组(n=45) 存活组(n=84) 统计值 P值 年龄(岁) 56.1±10.7 56.9±11.0 t=0.393 0.695 58.6±11.5 54.9±10.1 t=2.042 0.043 男性[例(%)] 60(68.9) 28(66.7) χ2=0.069 0.793 29(64.4) 59(70.2) χ2=0.454 0.501 肝硬化类型 乙型/丙型肝炎 45(51.7) 27(64.2) χ2=1.812 0.178 24(53.3) 48(57.1) χ2=0.172 0.678 酒精 23(26.4) 4(9.5) χ2=4.896 0.027 9(20.0) 18(21.4) χ2=0.036 0.849 其他 19(21.8) 11(26.2) χ2=0.301 0.584 12(26.7) 18(21.4) χ2=0.450 0.502 Child-Pugh评分(分) 7.1±1.3 7.2±1.4 t=0.542 0.588 7.4±1.5 7.0±1.2 t=1.604 0.111 Child-Pugh分级A/B(例) 37/50 16/26 χ2=0.230 0.632 14/31 39/45 χ2=2.840 0.092 感染[例(%)] 28(32.2) 13(30.9) χ2=0.513 0.474 24(53.3) 17(20.2) χ2=11.775 0.001 MAP(mm Hg) 86.5±10.8 87.3±10.6 t=0.355 0.723 82.4±10.2 89.2±10.4 t=3.177 0.002 WBC(×109/L) 3.4(2.5~4.9) 3.4(2.3~5.9) Z=-0.324 0.746 3.7(2.2~5.5) 3.4(2.4~4.9) Z=-1.613 0.107 中性粒细胞百分比 67.9±10.4 71.9±10.5 t=1.830 0.070 70.4±11.5 68.6±10.1 t=0.822 0.413 淋巴细胞百分比 16.5±9.6 16.0±9.8 t=0.224 0.823 12.7±10.1 17.9±9.1 t=2.598 0.011 Hb(g/L) 96.2±30.8 102.8±23.8 t=1.195 0.234 94.4±39.2 99.9±28.3 t=0.949 0.344 PLT(×109/L) 69.8±28.7 74.9±34.5 t=0.899 0.371 77.9±30.6 70.9±32.7 t=1.091 0.278 Alb(g/L) 32.2±5.7 32.8±5.5 t=0.740 0.461 33.4±6.5 33.3±5.3 t=0.080 0.937 ALT(U/L) 27(19~39.5) 23.5(16.8~47.5) Z=-0.458 0.647 27(18.25~48.7) 24(18~37) Z=-0.512 0.609 AST(U/L) 35(22~60) 38.5(24~65) Z=-0.641 0.522 27.5(47~78) 22(33.5~57.8) Z=-2.298 0.022 TBil(μmol/L) 18.4(11.7~27.9) 20.6(15.1~29.0) Z=-1.108 0.268 16.2(11.1~27.6) 19.8(13.7~29.6) Z=-0.074 0.941 尿素(mmol/L) 7.3±3.9 6.9±2.4 t=0.237 0.813 7.9±3.7 6.9±3.5 t=0.970 0.337 肌酐(μmol/L) 60.5±15.6 62.5±27.7 t=0.421 0.676 64.6±25.8 59.8±17.5 t=1.182 0.239 PTA 65.2±15.7 67.2±16.0 t=0.664 0.508 67.6±15.1 65.1±16.1 t=0.761 0.448 INR 1.4±0.3 1.4±0.3 t=0.456 0.649 1.4±0.3 1.4±0.3 t=0.783 0.435 门静脉内径(mm) 14.4±1.4 13.9±1.3 t=2.203 0.030 14.7±1.6 13.9±1.3 t=2.156 0.034 脾脏厚度(mm) 54.9±14.1 55.4±12.7 t=0.200 0.842 51.8±16.6 56.3±12.1 t=1.440 0.156 LSM(Kpa) 24.2(15.4~45) 19.0(13.5~24.7) Z=-2.771 0.006 21.3(14.3~41.5) 21.3(14.3~41.5) Z=-0.456 0.648 SSM(Kpa) 71.4±7.9 64.8±14.7 t=2.678 0.010 70.9±8.1 68.5±11.9 t=1.089 0.278 出血次数 - - - - 3.4±2.7 2.4±1.6 t=2.115 0.040 死亡[例(%)] 33(37.9) 12(28.6) χ2=1.092 0.296 - - - - 套扎治疗/联合治疗(例) 13/74 5/37 χ2=0.218 0.641 7/38 11/73 χ2=0.148 0.701 注:-, 无对应数据。 表 2 重度食管胃底静脉曲张出血患者再出血危险因素
影响因素 B SE WALD OR 95%CI P值 酒精性肝硬化 1.738 0.778 4.992 5.687 1.230~26.129 0.025 门静脉内径 0.246 0.163 2.296 1.279 0.930~1.759 0.129 LSM 0.039 0.015 7.051 1.039 1.010~1.070 0.007 SSM 0.075 0.024 9.734 1.078 1.028~1.129 0.001 表 3 重度EVB患者5年预后的危险因素
影响因素 B SE WALD OR 95%CI P值 门静脉内径 0.378 0.165 5.255 1.459 1.056~2.014 0.022 年龄 0.052 0.023 4.963 1.053 1.006~1.103 0.026 出血次数 0.252 0.109 5.373 1.286 1.040~1.591 0.020 MAP -0.019 0.022 0.740 0.981 0.939~1.025 0.390 淋巴细胞百分比 -0.028 0.023 1.480 0.972 0.929~1.017 0.224 AST 0.001 0.003 0.061 1.001 0.996~1.006 0.805 首次出血合并感染 1.656 0.558 8.806 5.239 1.750~15.641 0.003 -
[1] GARCIA-TSAO G, ABRALDES JG, BERZIGOTTI A, et al. Portal hypertensive bleeding in cirrhosis: Risk stratification, diagnosis, and management: 2016 practice guidance by the American Association for the study of liver diseases[J]. Hepatology, 2017, 65(1): 310-335. DOI: 10.1002/hep.28906. [2] Chinese Society of Hepatology, Chinese Medical Association; Chinese Society of Gastroenterology, Chinese Medical Association; Chinese Society of Endoscopy, Chinese Medical Association. Guidelines for the diagnosis and treatment of esophageal and gastric variceal bleeding in cirrhotic portal hypertension[J]. J Clin Hepatol, 2016, 32(2): 203-219. DOI: 10.3969/j.issn.1001-5256.2016.02.002.中华医学会肝病学分会, 中华医学会消化病学分会, 中华医学会内镜学分会. 肝硬化门静脉高压食管胃静脉曲张出血的防治指南[J]. 临床肝胆病杂志, 2016, 32(2): 203-219. DOI: 10.3969/j.issn.1001-5256.2016.02.002. [3] BOSCH J, GARCíA-PAGÁN JC. Prevention of variceal rebleeding[J]. Lancet, 361(9361): 952-954. DOI: 10.1016/S0140-6736(03)12778-X. [4] de FRANCHIS R, BAVENO VI Faculty. Expanding consensus in portal hypertension: Report of the Baveno VI Consensus Workshop: Stratifying risk and individualizing care for portal hypertension[J]. J Hepatol, 2015, 63(3): 743-752. DOI: 10.1016/j.jhep.2015.05.022. [5] Chinese Society of Hepatology, Chinese Medical Association. Chinese guidelines on the management of liver cirrhosis[J]. J Clin Hepatol, 2019, 35(11): 2408-2425. DOI: 10.3969/j.issn.1001-5256.2019.11.006.中华医学会肝病学分会. 肝硬化诊治指南[J]. 临床肝胆病杂志, 2019, 35(11): 2408-2425. DOI: 10.3969/j.issn.1001-5256.2019.11.006. [6] Committee of Esophageal Varicosity, Society of Digestive Endoscopy Chinese Medical Association. Tentative guidelines endoscopic diagnosis and treatment of varicosity and variceal bleeding in digestive tract (2009)[J]. Chin J Dig Endosc, 2010, 27(1): 1-4. DOI: 10.3760/cma.j.issn.1007-5232.2010.01.001.中华医学会消化内镜学分会食管胃静脉曲张学组. 消化道静脉曲张及出血的内镜诊断和治疗规范试行方案(2009年)[J]. 中华消化内镜杂志, 2010, 27(1): 1-4. DOI: 10.3760/cma.j.issn.1007-5232.2010.01.001. [7] SHARMA P, SARIN SK. Improved survival with the patients with variceal bleed[J]. Int J Hepatol, 2011, 2011: 356919. DOI: 10.4061/2011/356919. [8] Chinese Portal Hypertension Diagnosis and Monitoring Study Group(CHESS); Minimally Invasive Intervention Collaborative Group, Chinese Society of Gastroenterology; Emergency Intervention Committee, Chinese College of Interventionalists, et al. Consensus on clinical application of hepatic venous pressure gradient in China (2018)[J]. J Clin Hepatol, 2018, 34(12): 2526-2536. DOI: 10.3969/j.issn.1001-5256.2018.12.008中国门静脉高压诊断与监测研究组(CHESS), 中华医学会消化病学分会微创介入协作组, 中国医师协会介入医师分会急诊介入专业委员会, 等. 中国肝静脉压力梯度临床应用专家共识(2018版)[J]. 临床肝胆病杂志, 2018, 34(12): 2526-2536. DOI: 10.3969/j.issn.1001-5256.2018.12.008 [9] ABD EL RIHIM AY, OMAR RF, FATHALAH W, et al. Role of fibroscan and APRI in detection of liver fibrosis: A systematic review and meta-analysis[J]. Arab J Gastroenterol, 2013, 14(2): 44-50. DOI: 10.1016/j.ajg.2013.05.002. [10] BUREAU C, METIVIER S, PERON JM, et al. Transient elastography accurately predicts presence of significant portal hypertension in patients with chronic liver disease[J]. Aliment Pharmacol Ther, 2008, 27(12): 1261-1268. DOI: 10.1111/j.1365-2036.2008.03701.x. [11] LIU F, LI TH, HAN T, et al. Non-invasive assessment of portal hypertension in patients with liver cirrhosis using FibroScan transient elastography[J]. Chin J Hepatol, 2013, 21(11): 840- 844. DOI: 10.3760/cma.j.issn.1007-3418.2013.11.010.刘芳, 李庭红, 韩涛, 等. 瞬时弹性成像在肝硬化门静脉高压中的临床评价[J]. 中华肝脏病杂志, 2013, 21(11): 840-844. DOI: 10.3760/cma.j.issn.1007-3418.2013.11.010. [12] LI TH, LIU F, HAN T, et al. Noninvasive assessment of esophageal-gastric varices by spleen stiffiness liver cirrhosis patients[J]. Chin J Infect, 2012, 30(10): 603-608. DOI: 10.3760/cma.j.issn.1000-6680.2012.10.006.李庭红, 刘芳, 韩涛, 等. 脾脏硬度对肝硬化患者食管胃底静脉曲张的评估[J]. 中华传染病杂志, 2012, 30(10): 603-608. DOI: 10.3760/cma.j.issn.1000-6680.2012.10.006. [13] COLECCHIA A, MONTRONE L, SCAIOLI E, et al. Measurement of spleen stiffness to evaluate portal hypertension and the presence of esophageal varices in patients with HCV-related cirrhosis[J]. Gastroenterology, 2012, 143(3): 646-654. DOI: 10.1053/j.gastro.2012.05.035. [14] TAKUMA Y, NOUSO K, MORIMOTO Y, et al. Portal hypertension in patients with liver cirrhosis: Diagnostic accuracy of spleen stiffness[J]. Radiology, 2016, 279(2): 609-619. DOI: 10.1148/radiol.2015150690. [15] SHARMA P, KIRNAKE V, TYAGI P, et al. Spleen stiffness in patients with cirrhosis in predicting esophageal varices[J]. Am J Gastroenterol, 2013, 108(7): 1101-1107. DOI: 10.1038/ajg.2013.119. [16] NILLES KM, FLAMM SL. Thrombocytopenia in chronic liver disease: New management strategies[J]. Clin Liver Dis, 2020, 24(3): 437-451. DOI: 10.1016/j.cld.2020.04.009. [17] MEJIAS M, GARCIA-PRAS E, GALLEGO J, et al. Relevance of the mTOR signaling pathway in the pathophysiology of splenomegaly in rats with chronic portal hypertension[J]. J Hepatol, 2010, 52(4): 529-539. DOI: 10.1016/j.jhep.2010.01.004. [18] LAMEIRÄO GOMES C, VIOLANTE SILVA R, CARROLA P, et al. Bacterial infections in patients with liver cirrhosis in an internal medicine department[J]. GE Port J Gastroenterol, 2019, 26(5): 324-332. DOI: 10.1159/000494568. [19] GELETO G, GETNET W, TEWELDE T. Mean normal portal vein diameter using sonography among clients coming to radiology department of Jimma university hospital, Southwest Ethiopia[J]. Ethiop J Health Sci, 2016, 26(3): 237-242. DOI: 10.4314/ejhs.v26i3.6. [20] TANEJA SK, DHIMAN RK. Prevention and management of bacterial infections in cirrhosis[J]. Int J Hepatol, 2011, 2011: 784540. DOI: 10.4061/2011/784540. [21] Ali A, Swingland J, Choi CH, et al. OC-143 Artificial neural network for the risk stratification of acute upper gastrointestinal bleeding: multicentre comparative analysis vs the Glasgow Blatchford and rockall scores[J]. Gut, 2012, 61(Suppl 2): A62. DOI: 10.1136/gutjnl-2012-302514a.143. [22] FERNANDEZ J, ARROYO V. Bacterial infections in cirrhosis: A growing problem with significant implications[J]. Clin Liver Dis (Hoboken), 2013, 2(3): 102-105. DOI: 10.1002/cld.169. [23] PARK EJ, JANG JY, LEE JE, et al. The risk factors for bleeding of fundal varices in patients with liver cirrhosis[J]. Gut Liver, 2013, 7(6): 704-711. DOI: 10.5009/gnl.2013.7.6.704. 期刊类型引用(9)
1. 陈玉凤,明文,陈镜晶,王茜,李溢馨. FAR、RLR水平对乙型肝炎肝硬化并发食管胃底静脉曲张破裂出血患者治疗后再出血风险的评估价值. 现代生物医学进展. 2025(01): 72-79 .  百度学术
百度学术2. 赵孟杰,邢恩涛,董源,金鹏飞,孙景武. 不可逆电穿孔和射频消融治疗原发性肝癌效果比较. 青岛大学学报(医学版). 2024(01): 110-114 .  百度学术
百度学术3. 谢亚明,梁磊,肖遵强,刘军伟,张成武,黄东胜. Naples预后评分对肝细胞癌患者肝切除术后预后的影响. 中华肝胆外科杂志. 2024(05): 341-346 .  百度学术
百度学术4. 叶建刚,彭俊文,李莉. 乳腺X线和MRI参数联合外周血RLR水平预测乳腺癌改良根治术后复发转移的价值. 中华内分泌外科杂志. 2024(03): 404-408 .  百度学术
百度学术5. 张玉霞,谢钦,魏思瑞,姜龙琳,谢丽,韩泳涛,缪艳. 炎症及营养指标对老年食管鳞癌患者术后生存的预测价值. 中华消化外科杂志. 2024(09): 1200-1208 .  百度学术
百度学术6. 张兰,李胜棉. 《肝癌新辅助治疗中国专家共识(2023版)》专家解读. 疑难病杂志. 2024(11): 1281-1286 .  百度学术
百度学术7. 潘伟华,黄腾钦,曾耿华. 经皮微波消融术和腹腔镜肝癌切除术治疗小肝癌的临床效果. 中国医学创新. 2023(17): 17-20 .  百度学术
百度学术8. 张旭,哈福双,李凤惠,高艳颖,梁静. 肝纤维化-4指数(FIB-4)联合预后营养指数(PNI)对早期肝癌射频消融术后复发及生存期的预测价值. 临床肝胆病杂志. 2023(11): 2614-2622 .  本站查看
本站查看9. 陈雪岩,乔建梁,李军,牛剑祥,赵建国,韩赛,孟兴凯. 小野寺预后营养指数对消化系统恶性肿瘤预后预测价值的研究进展. 中华消化外科杂志. 2022(10): 1390-1394 .  百度学术
百度学术其他类型引用(0)
-




 PDF下载 ( 2086 KB)
PDF下载 ( 2086 KB)


 下载:
下载:



 下载:
下载:
 百度学术
百度学术