肝癌细胞来源外泌体对肿瘤相关M2型巨噬细胞极化的影响
DOI: 10.3969/j.issn.1001-5256.2022.03.013
利益冲突声明:本研究不存在研究者、伦理委员会成员、受试者监护人以及与公开研究成果有关的利益冲突。
作者贡献声明:姚涛、徐值红参与课题设计,资料分析,撰写论文;姚纪友、夏雨参与收集数据,修改论文;刘冰、兰天、陆敏强负责课题设计,数据资料分析,指导撰写文章并最后定稿。
Effect of hepatocellular carcinoma cell-derived exosomes on M2 polarization of tumor-associated macrophages
-
摘要:
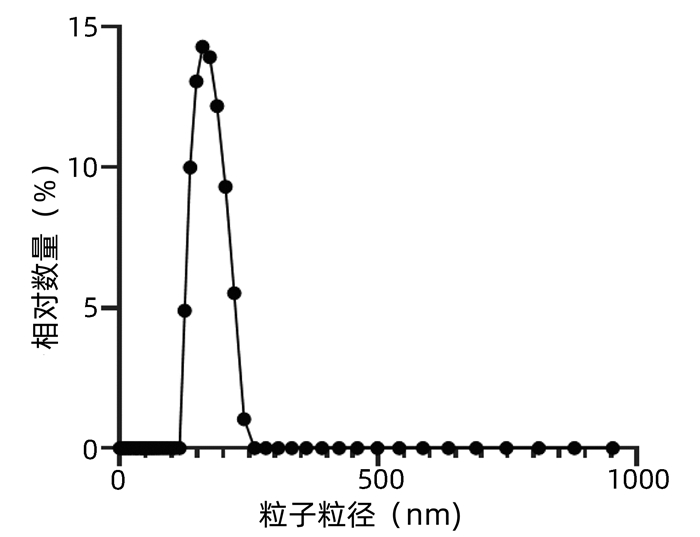
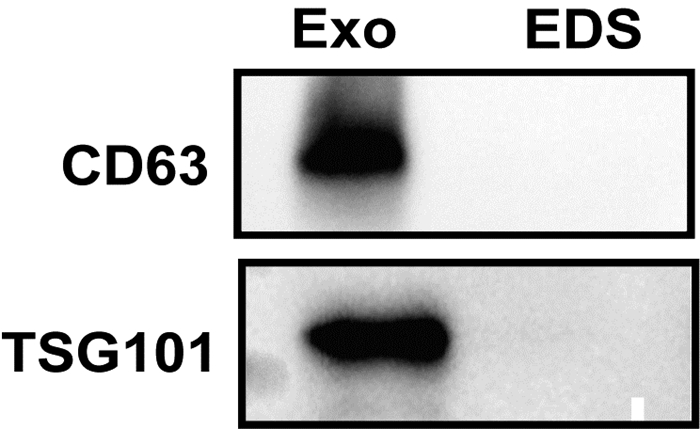
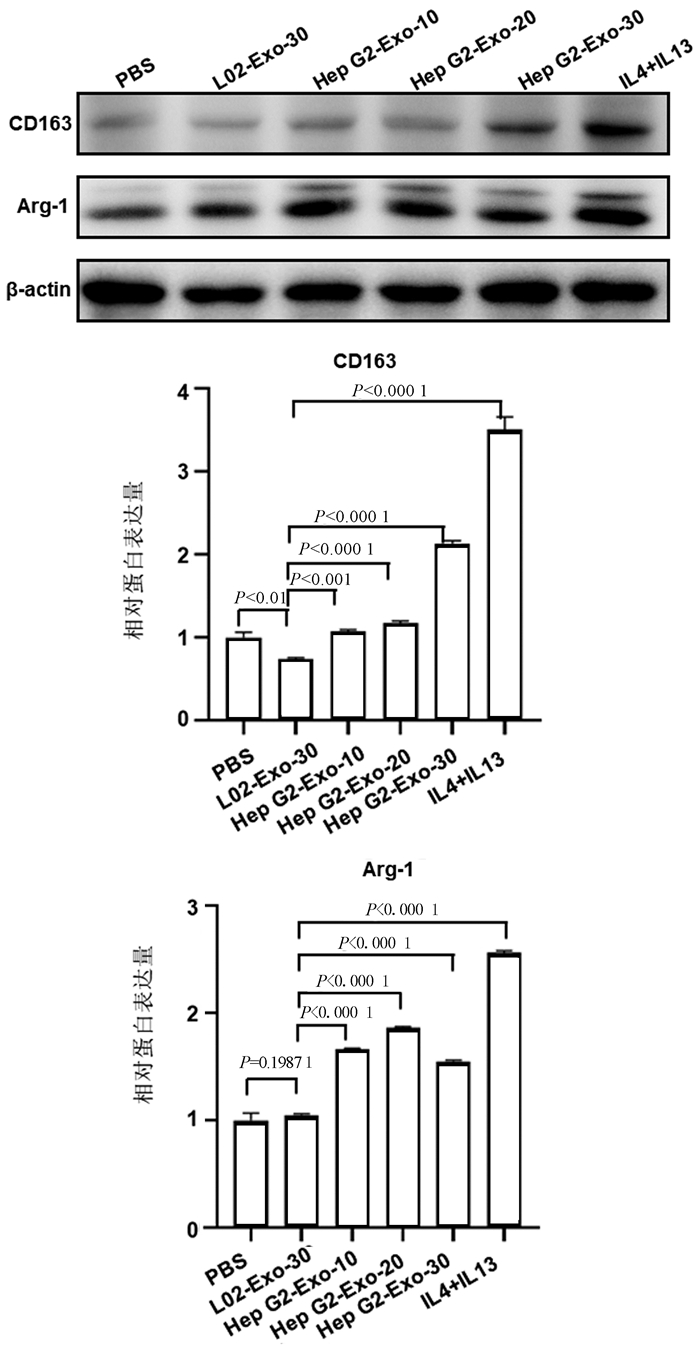
目的 探讨来源于肝癌细胞的外泌体对肿瘤相关巨噬细胞极化的影响,揭示肝癌形成新机制。 方法 通过超速离心法分离肝癌细胞来源外泌体,透射电子显微镜、动态光散射粒度分析仪、蛋白免疫印迹法对外泌体表征进行鉴定;诱导巨噬细胞极化模型,实时荧光定量PCR和蛋白免疫印迹法验证其极化状态。符合正态分布的计量资料两组间比较采用t检验;多组间比较采用单因素方差分析,进一步两两比较采用LSD-t检验。 结果 透谢电子显微镜显示肝癌细胞来源外泌体为圆形或椭圆形囊泡结构,外泌体粒径大小为(172.65±2.34)nm,蛋白免疫印迹分析显示肝癌细胞来源的外泌体中标志蛋白TSG101和CD63呈高阳性表达。佛波酯15 ng诱导人源单核细胞巨噬细胞24 h贴壁后CD68表达显著增加(6.67±0.98 vs 1.00±0.25,t=11.20,P<0.001)。蛋白免疫印迹分析显示,相比对照组(L02来源外泌体组),HCC细胞来源外泌体(低、中、高3种剂量)诱导巨噬细胞表达M2型巨噬细胞标志物Arg-1、CD163均明显增加(P值均<0.05)。 结论 肝癌细胞来源外泌体可促进巨噬细胞M2型极化。 Abstract:Objective To investigate the effect of exosomes derived from hepatocellular carcinoma cells on the polarization of tumor-associated macrophages (TAMs), and to reveal the novel mechanism of hepatocellular carcinoma formation. Methods Hepatocellular carcinoma cell-derived exosomes were isolated by ultracentrifugation, and the characteristics of exosomes were identified by transmission electron microscope (TEM), Dynamic Light Scattering (DLS), and Western blotting. The model of macrophage polarization was induced and verified by quantitative real-time PCR and Western blotting. The t-test was used for comparison of normally distributed continuous data between two groups. A one-way analysis of variance was used for comparison between multiple groups, and the LSD-t-test was used for further comparison between two groups. Results TEM showed that hepatocellular carcinoma cell-derived exosomes were round or oval vesicles, LDS showed that the exosomes had a particle size of 172.65±2.34 nm, and Western blotting showed highly positive expression of the biomarkers TSG101 and CD63 in exosomes. There was a significant increase in the expression of CD68 after the addition of 15 ng phorbol ester to induce human-derived mononuclear macrophages for 24 hours to achieve adherent growth (1.00±0.25 vs 6.67±0.98, t=11.20, P < 0.001). Western blotting showed that compared with the control group (L02 cell-derived exosomes), the hepatocellular carcinoma cell-derived exosomes (at low, middle, and high doses) induced M2 polarization of macrophages and increased the expression of the markers Arg-1 and CD163 (all P < 0.05). Conclusion Hepatocellular carcinoma cell-derived exosomes promote M2 polarization of TAMs. -
Key words:
- Carcinoma, Hepatocellular /
- Exosomes /
- Macrophages
-
[1] CHEN R, XU X, TAO Y, et al. Exosomes in hepatocellular carcinoma: A new horizon[J]. Cell Commun Signal, 2019, 17(1): 1. DOI: 10.1186/s12964-018-0315-1. [2] VILLANUEVA A. Hepatocellular carcinoma[J]. N Engl J Med, 2019, 380(15): 1450-1462. DOI: 10.1056/NEJMra1713263. [3] ROCHE B, COILLY A, DUCLOS-VALLEE JC, et al. The impact of treatment of hepatitis C with DAAs on the occurrence of HCC[J]. Liver Int, 2018, 38(Suppl 1): 139-145. DOI: 10.1111/liv.13659. [4] WU Y, ZHANG J, ZHANG X, et al. Cancer stem cells: A potential breakthrough in HCC-targeted therapy[J]. Front Pharmacol, 2020, 11: 198. DOI: 10.3389/fphar.2020.00198. [5] TACKE F. Targeting hepatic macrophages to treat liver diseases[J]. J Hepatol, 2017, 66(6): 1300-1312. DOI: 10.1016/j.jhep.2017.02.026. [6] WU J, GAO W, TANG Q, et al. M2 macrophage-derived exosomes facilitate HCC metastasis by transferring α(M) β(2) integrin to tumor cells[J]. Hepatology, 2021, 73(4): 1365-1380. DOI: 10.1002/hep.31432. [7] LI W, NG JM, WONG CC, et al. Molecular alterations of cancer cell and tumour microenvironment in metastatic gastric cancer[J]. Oncogene, 2018, 37(36): 4903-4920. DOI: 10.1038/s41388-018-0341-x. [8] NGAMBENJAWONG C, GUSTAFSON HH, PUN SH. Progress in tumor-associated macrophage (TAM)-targeted therapeutics[J]. Adv Drug Deliv Rev, 2017, 114: 206-221. DOI: 10.1016/j.addr.2017.04.010. [9] HINSHAW DC, SHEVDE LA. The tumor microenvironment innately modulates cancer progression[J]. Cancer Res, 2019, 79(18): 4557-4566. DOI: 10.1158/0008-5472.CAN-18-3962. [10] PEGTEL DM, GOULD SJ. Exosomes[J]. Annu Rev Biochem, 2019, 88: 487-514. DOI: 10.1146/annurev-biochem-013118-111902. [11] HARDING C, HEUSER J, STAHL P. Receptor-mediated endocytosis of transferrin and recycling of the transferrin receptor in rat reticulocytes[J]. J Cell Biol, 1983, 97(2): 329-339. DOI: 10.1083/jcb.97.2.329. [12] PAN BT, JOHNSTONE RM. Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: Selective externalization of the receptor[J]. Cell, 1983, 33(3): 967-978. DOI: 10.1016/0092-8674(83)90040-5. [13] PURUSHOTHAMAN A, BANDARI SK, LIU J, et al. Fibronectin on the surface of myeloma cell-derived exosomes mediates exosome-cell interactions[J]. J Biol Chem, 2016, 291(4): 1652-1663. DOI: 10.1074/jbc.M115.686295. [14] WANG F, LI L, PIONTEK K, et al. Exosome miR-335 as a novel therapeutic strategy in hepatocellular carcinoma[J]. Hepatology, 2018, 67(3): 940-954. DOI: 10.1002/hep.29586. [15] QIAN M, WANG S, GUO X, et al. Hypoxic glioma-derived exosomes deliver microRNA-1246 to induce M2 macrophage polarization by targeting TERF2IP via the STAT3 and NF-κB pathways[J]. Oncogene, 2020, 39(2): 428-442. DOI: 10.1038/s41388-019-0996-y. [16] LI QF, XIA T, SHI CW, et al. Effects of exosomes secreted by hepatocellular carcinoma cells under hypoxic environment on homologous cell activity[J]. Chin J Gerontol, 2022, 42(3): 672-675.李全富, 夏天, 石春薇, 等. 肝癌细胞低氧环境下分泌的外泌体对同源细胞活性的影响[J]. 中国老年学杂志, 2022, 42(3): 672-675. [17] ZHAO S, MI Y, GUAN B, et al. Tumor-derived exosomal miR-934 induces macrophage M2 polarization to promote liver metastasis of colorectal cancer[J]. J Hematol Oncol, 2020, 13(1): 156. DOI: 10.1186/s13045-020-00991-2. [18] FANG T, LV H, LV G, et al. Tumor-derived exosomal miR-1247-3p induces cancer-associated fibroblast activation to foster lung metastasis of liver cancer[J]. Nat Commun, 2018, 9(1): 191. DOI: 10.1038/s41467-017-02583-0. [19] LOU G, CHEN Z, ZHENG M, et al. Mesenchymal stem cell-derived exosomes as a new therapeutic strategy for liver diseases[J]. Exp Mol Med, 2017, 49(6): e346. DOI: 10.1038/emm.2017.63. [20] GANDHAM S, SU X, WOOD J, et al. Technologies and standardization in research on extracellular vesicles[J]. Trends Biotechnol, 2020, 38(10): 1066-1098. DOI: 10.1016/j.tibtech.2020.05.012. [21] TI D, HAO H, TONG C, et al. LPS-preconditioned mesenchymal stromal cells modify macrophage polarization for resolution of chronic inflammation via exosome-shuttled let-7b[J]. J Transl Med, 2015, 13: 308. DOI: 10.1186/s12967-015-0642-6. 期刊类型引用(9)
1. 刘韦,白浪. 慢加急性肝衰竭动物模型研究现状. 临床肝胆病杂志. 2024(01): 187-192 .  本站查看
本站查看2. 陈然,王帅,高扬,杨志琴,徐严. HBV相关慢加急性肝衰竭合并脓毒症的诊疗进展. 肝脏. 2024(07): 867-870 .  百度学术
百度学术3. 刘扬,洪文,黄克林,杨博,刘亚坡,路明. 粪菌移植对顽固性便秘小鼠的肠道菌群和肠道动力以及TLR4/NF-κB通路蛋白的影响. 现代生物医学进展. 2024(16): 3020-3024 .  百度学术
百度学术4. 于丹丹,刘亚坡,洪文,杨博,张媛. 粪菌移植对顽固性便秘患者肠道功能、免疫功能及炎症反应的改善效果. 结直肠肛门外科. 2024(05): 578-583 .  百度学术
百度学术5. 阮浩龙,王宁,于鸿浩,岳鹏鹏. 基于基因编辑技术研究特定基因对小鼠肠道微生物的影响. 中国医学工程. 2024(11): 44-49 .  百度学术
百度学术6. 徐洪凯,汪春付,张野,连建奇. 粪菌移植在慢性肝病治疗中的应用. 临床肝胆病杂志. 2023(09): 2237-2243 .  本站查看
本站查看7. 周荃,蔡春琳,李金强. 肠-肝轴:肠道微生物稳态与肝细胞癌. 临床肝胆病杂志. 2023(11): 2710-2717 .  本站查看
本站查看8. 周宜,刘晓琴,吴学敏,张浩,王海琴,王红亮,孟祥龙. 乳香及其炮制品对葡聚糖硫酸钠诱导小鼠炎症性肠病的保护作用. 现代药物与临床. 2022(07): 1432-1438 .  百度学术
百度学术9. 刘蕾,高越颖,郭琳,邱立朋,李会,耿燕. 酒精性肝损伤保护的药理实验教学设计. 实验室研究与探索. 2022(05): 238-243 .  百度学术
百度学术其他类型引用(2)
-




 PDF下载 ( 2353 KB)
PDF下载 ( 2353 KB)


 下载:
下载:
 百度学术
百度学术

