| [1] |
Chinese Society of Infectious Diseases, Chinese Medical Association. Guidelines for the prevention and treatment of chronic hepatitis B(version 2019)[J]. J Clin Hepatol, 2019, 35( 12): 2648- 2669. DOI: 10.3969/j.issn.1001-5256.2019.12.007. |
| [2] |
LIU J, LIANG WN, JING WZ, et al. Countdown to 2030: Eliminating hepatitis B disease, China[J]. Bull World Health Organ, 2019, 97( 3): 230- 238. DOI: 10.2471/BLT.18.219469. |
| [3] |
PETERSEN J, THOMPSON AJ, LEVRERO M. Aiming for cure in HBV and HDV infection[J]. J Hepatol, 2016, 65( 4): 835- 848. DOI: 10.1016/j.jhep.2016.05.043. |
| [4] |
TIAN Y, REN F. Advances in the detection and clearance of hepatitis B virus covalently closed circular DNA(cccDNA)[J]. Chin J Microbiol Immunol, 2019, 39( 11): 875- 879. DOI: 10.3760/cma.j.issn.0254-5101.2019.11.011. |
| [5] |
ZAI WJ, CHEN JL, YUAN ZH. Regulatory mechanisms of the transcription and metabolism of hepatitis B virus covalently closed circular DNA and strategies for silencing and elimination[J]. J Clin Hepatol, 2020, 36( 5): 983- 988. DOI: 10.3969/j.issn.1001-5256.2020.05.006. |
| [6] |
ZHANG Y, ZHAO JM. Clinical application of covalently closed circular DNA detection techniques[J]. J Clin Hepatol, 2019, 35( 10): 2140- 2144. DOI: 10.3969/j.issn.1001-5256.2019.10.002. |
| [7] |
|
| [8] |
WANG J, SHEN T, HUANG XB, et al. Serum hepatitis B virus RNA is encapsidated pregenome RNA that may be associated with persistence of viral infection and rebound[J]. J Hepatol, 2016, 65( 4): 700- 710. DOI: 10.1016/j.jhep.2016.05.029. |
| [9] |
XIA MY, CHI H, WU YB, et al. Serum hepatitis B virus RNA level is associated with biochemical relapse in patients with chronic hepatitis B infection who discontinue nucleos(t)ide analogue treatment[J]. Aliment Pharmacol Ther, 2021, 54( 5): 709- 714. DOI: 10.1111/apt.16538. |
| [10] |
LU FM, WANG J, CHEN XM, et al. The potential use of serum HBV RNA to guide the functional cure of chronic hepatitis B[J]. Chin J Hepatol, 2017, 25( 2): 105- 110. DOI: 10.3760/cma.j.issn.1007-3418.2017.02.005. |
| [11] |
KÖCK J, THEILMANN L, GALLE P, et al. Hepatitis B virus nucleic acids associated with human peripheral blood mononuclear cells do not originate from replicating virus[J]. Hepatology, 1996, 23( 3): 405- 413. DOI: 10.1002/hep.510230303. |
| [12] |
TAO YC, WANG ML, LIAO J, et al. Dynamics of serum pregenome RNA in chronic hepatitis B patients receiving 96-month nucleos(t)ide analog therapy[J]. Front Med, 2022, 9: 787770. DOI: 10.3389/fmed.2022.787770. |
| [13] |
ZHANG X, AN XC, SHI L, et al. Baseline quantitative HBcAb strongly predicts undetectable HBV DNA and RNA in chronic hepatitis B patients treated with entecavir for 10 years[J]. Sci Rep, 2021, 11( 1): 13389. DOI: 10.1038/s41598-021-92757-0. |
| [14] |
SHEN S, XIE ZL, CAI DW, et al. Biogenesis and molecular characteristics of serum hepatitis B virus RNA[J]. PLoS Pathog, 2020, 16( 10): e1008945. DOI: 10.1371/journal.ppat.1008945. |
| [15] |
PRAKASH K, RYDELL GE, LARSSON SB, et al. High serum levels of pregenomic RNA reflect frequently failing reverse transcription in hepatitis B virus particles[J]. Virol J, 2018, 15( 1): 86. DOI: 10.1186/s12985-018-0994-7. |
| [16] |
|
| [17] |
LI H, XU WT, DENG BC, et al. Research progress of nucleoside(acid) analogues combined with pegylated interferon in functional treatment of chronic hepatitis B[J]. Clin J Med Offic, 2022, 50( 9): 890- 893. DOI: 10.16680/j.1671-3826.2022.09.04. |
| [18] |
GIERSCH K, ALLWEISS L, VOLZ T, et al. Serum HBV pgRNA as a clinical marker for cccDNA activity[J]. J Hepatol, 2017, 66( 2): 460- 462. DOI: 10.1016/j.jhep.2016.09.028. |
| [19] |
WANG Y, LIU YN, LIAO H, et al. Serum HBV DNA plus RNA reflecting cccDNA level before and during NAs treatment in HBeAg positive CHB patients[J]. Int J Med Sci, 2022, 19( 5): 858- 866. DOI: 10.7150/ijms.71737. |
| [20] |
PENG YM, YUAN H, ZHOU YF, et al. Serum hepatitis B virus RNA level in chronic hepatitis B with low level of hepatitis B virus DNA and its influential factors[J]. Chin Gen Pract, 2019, 22( 18): 2217- 2222. DOI: 10.12114/j.issn.1007-9572.2019.00.020. |
| [21] |
WANG CK, LIU SR. Expression level of serum HBV RNA in HBeAg-positive chronic hepatitis B patients at different periods and its value of measurement[J]. J Clin Hepatol, 2021, 37( 12): 2798- 2801. DOI: 10.3969/j.issn.1001-5256.2021.12.014. |
| [22] |
LING XZ, WANG RM, SU MH, et al. Clinical significance of dynamic monitoring of serum HBV pgRNA in CHB patients treated with NAs[J]. Chin J Pract Intern Med, 2022, 42( 5): 404- 408. DOI: 10.19538/j.nk2022050112. 零小樟, 王荣明, 苏明华, 等. 慢性乙型肝炎患者核苷酸类似物治疗中动态监测血清乙型肝炎病毒前基因组RNA的临床意义[J]. 中国实用内科杂志, 2022, 42( 5): 404- 408. DOI: 10.19538/j.nk2022050112. |
| [23] |
ZHU Y, LUO YX, GUO FX, et al. Predictive value of serum HBV RNA for therapeutic effect of entecavir in patients with chronic hepatitis B[J]. J South Med Univ, 2022, 42( 8): 1250- 1255. DOI: 10.12122/j.issn.1673-4254.2022.08.19. |
| [24] |
JIANG B, DAI QH, LIU YM, et al. Levels of HBV RNA in chronic HBV infected patients during first-line nucleos(t)ide analogues therapy[J]. Infect Agent Cancer, 2022, 17( 1): 61. DOI: 10.1186/s13027-022-00473-9. |



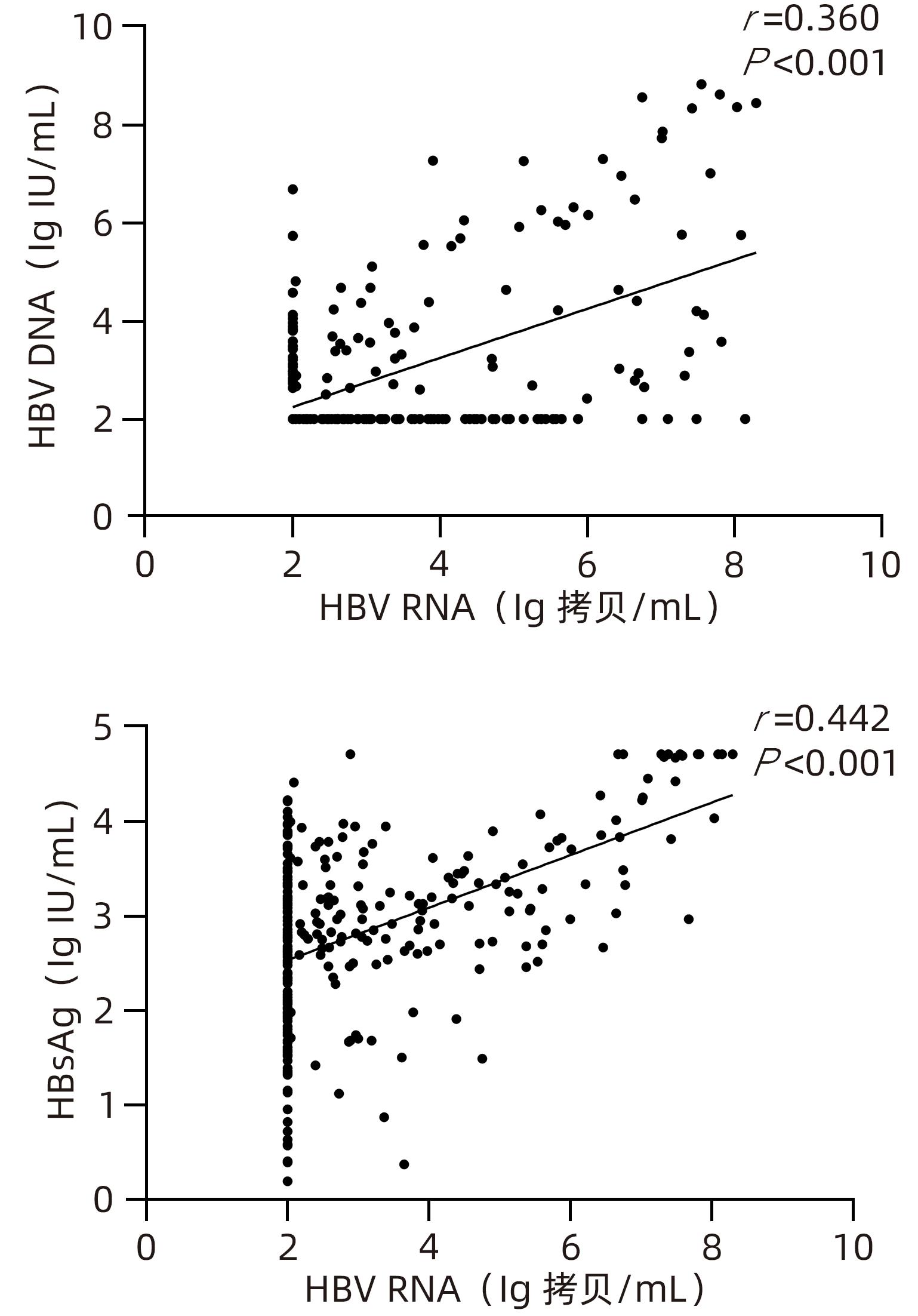




 DownLoad:
DownLoad: