| [1] |
LOCKRIDGE O. Review of human butyrylcholinesterase structure, function, genetic variants, history of use in the clinic, and potential therapeutic uses[J]. Pharmacol Ther, 2015, 148: 34- 46. DOI: 10.1016/j.pharmthera.2014.11.011. |
| [2] |
ZHU GD, DAWSON E, HUSKEY A, et al. Genetic testing for BCHE variants identifies patients at risk of prolonged neuromuscular blockade in response to succinylcholine[J]. Pharmgenomics Pers Med, 2020, 13: 405- 414. DOI: 10.2147/PGPM.S263741. |
| [3] |
IIDA S, KINOSHITA M, FUJII H, et al. Mutations of human butyrylcholinesterase gene in a family with hypocholinesterasemia[J]. Hum Mutat, 1995, 6( 4): 349- 351. DOI: 10.1002/humu.1380060411. |
| [4] |
YEN T, NIGHTINGALE BN, BURNS JC, et al. Butyrylcholinesterase(BCHE) genotyping for post-succinylcholine apnea in an Australian population[J]. Clin Chem, 2003, 49( 8): 1297- 1308. DOI: 10.1373/49.8.1297. |
| [5] |
YU RT, GUO YZ, DAN YJ, et al. A novel mutation in the BCHE gene and phenotype identified in a child with low butyrylcholinesterase activity: A case report[J]. BMC Med Genet, 2018, 19( 1): 58. DOI: 10.1186/s12881-018-0561-5. |
| [6] |
MANOHARAN I, BOOPATHY R, DARVESH S, et al. A medical health report on individuals with silent butyrylcholinesterase in the Vysya community of India[J]. Clin Chim Acta, 2007, 378( 1-2): 128- 135. DOI: 10.1016/j.cca.2006.11.005. |
| [7] |
LOCKRIDGE O, NORGREN RB Jr, JOHNSON RC, et al. Naturally occurring genetic variants of human acetylcholinesterase and butyrylcholinesterase and their potential impact on the risk of toxicity from cholinesterase inhibitors[J]. Chem Res Toxicol, 2016, 29( 9): 1381- 1392. DOI: 10.1021/acs.chemrestox.6b00228. |
| [8] |
NICOLET Y, LOCKRIDGE O, MASSON P, et al. Crystal structure of human butyrylcholinesterase and of its complexes with substrate and products[J]. J Biol Chem, 2003, 278( 42): 41141- 41147. DOI: 10.1074/jbc.M210241200. |
| [9] |
AMMUNDSEN HB, SØRENSEN MK, GÄTKE MR. Succinylcholine resistance[J]. Br J Anaesth, 2015, 115( 6): 818- 821. DOI: 10.1093/bja/aev228. |
| [10] |
WICHMANN S, FÆRK G, BUNDGAARD JR, et al. Patients with prolonged effect of succinylcholine or mivacurium had novel mutations in the butyrylcholinesterase gene[J]. Pharmacogenet Genomics, 2016, 26( 7): 351- 356. DOI: 10.1097/FPC.0000000000000221. |
| [11] |
ANDERSSON ML, MØLLER AM, WILDGAARD K. Butyrylcholinesterase deficiency and its clinical importance in anaesthesia: A systematic review[J]. Anaesthesia, 2019, 74( 4): 518- 528. DOI: 10.1111/anae.14545. |
| [12] |
BRIMIJOIN S, TYE S. Favorable impact on stress-related behaviors by modulating plasma butyrylcholinesterase[J]. Cell Mol Neurobiol, 2018, 38( 1): 7- 12. DOI: 10.1007/s10571-017-0523-z. |
| [13] |
LI Q, YANG HY, CHEN Y, et al. Recent progress in the identification of selective butyrylcholinesterase inhibitors for Alzheimer’s disease[J]. Eur J Med Chem, 2017, 132: 294- 309. DOI: 10.1016/j.ejmech.2017.03.062. |
| [14] |
SANTARPIA L, GRANDONE I, CONTALDO F, et al. Butyrylcholinesterase as a prognostic marker: A review of the literature[J]. J Cachexia Sarcopenia Muscle, 2013, 4( 1): 31- 39. DOI: 10.1007/s13539-012-0083-5. |
| [15] |
SATO T, YAMAUCHI H, SUZUKI S, et al. Serum cholinesterase is an important prognostic factor in chronic heart failure[J]. Heart Vessels, 2015, 30( 2): 204- 210. DOI: 10.1007/s00380-014-0469-8. |
| [16] |
GOLIASCH G, KLEBER ME, RICHTER B, et al. Routinely available biomarkers improve prediction of long-term mortality in stable coronary artery disease: The Vienna and Ludwigshafen Coronary Artery Disease(VILCAD) risk score[J]. Eur Heart J, 2012, 33( 18): 2282- 2289. DOI: 10.1093/eurheartj/ehs164. |
| [17] |
YUAN M, HAN B, XIA YP, et al. Augmentation of peripheral lymphocyte-derived cholinergic activity in patients with acute ischemic stroke[J]. BMC Neurol, 2019, 19( 1): 236. DOI: 10.1186/s12883-019-1481-5. |
| [18] |
GREMMEL T, WADOWSKI PP, MUELLER M, et al. Serum cholinesterase levels are associated with 2-year ischemic outcomes after angioplasty and stenting for peripheral artery disease[J]. J Endovasc Ther, 2016, 23( 5): 738- 743. DOI: 10.1177/1526602816655521. |
| [19] |
FENG WM, TANG CW, GUO HH, et al. Prognostic value of serum cholinesterase activities in sepsis patients[J]. Hepatogastroenterology, 2013, 60( 125): 1001- 1005. DOI: 10.5754/hge13141. |
| [20] |
ZIVKOVIC AR, TOURELLE KM, BRENNER T, et al. Reduced serum cholinesterase activity indicates splenic modulation of the sterile inflammation[J]. J Surg Res, 2017, 220: 275- 283. DOI: 10.1016/j.jss.2017.07.024. |
| [21] |
KLOCKER EV, BARTH DA, RIEDL JM, et al. Decreased activity of circulating butyrylcholinesterase in blood is an independent prognostic marker in pancreatic cancer patients[J]. Cancers(Basel), 2020, 12( 5): 1154. DOI: 10.3390/cancers12051154. |
| [22] |
YAMAMOTO M, SAITO H, UEJIMA C, et al. Combination of serum albumin and cholinesterase levels as prognostic indicator in patients ith colorectal cancer[J]. Anticancer Res, 2019, 39( 2): 1085- 1090. DOI: 10.21873/anticanres.13217. |
| [23] |
SOUZA RL, MIKAMI LR, MAEGAWA RO, et al. Four new mutations in the BCHE gene of human butyrylcholinesterase in a Brazilian blood donor sample[J]. Mol Genet Metab, 2005, 84( 4): 349- 353. DOI: 10.1016/j.ymgme.2004.12.005. |
| [24] |
ON-KEI CHAN A, LAM CW, TONG SF, et al. Novel mutations in the BCHE gene in patients with no butyrylcholinesterase activity[J]. Clin Chim Acta, 2005, 351( 1-2): 155- 159. DOI: 10.1016/j.cccn.2004.09.004. |
| [25] |
LV HY, YANG LH, BU LN, et al. A case report of primary neonatal hypocholinesterase caused by homozygous frameshift mutation of the utyrylcholinesterase(BCHE) gene and review of literature[J]. Clin Lab, 2019, 65( 7). DOI: 10.7754/Clin.Lab.2019.181254. |



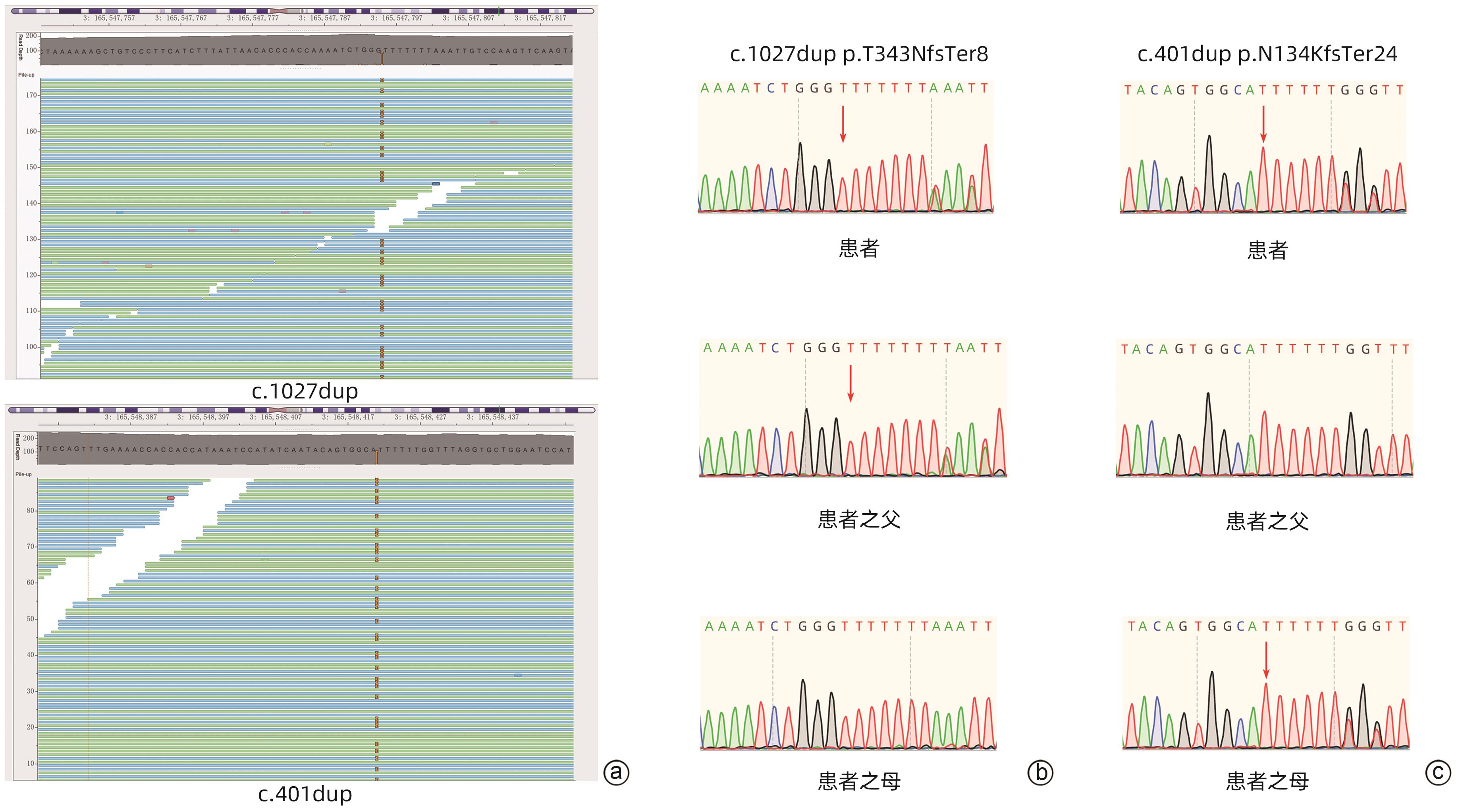




 DownLoad:
DownLoad: