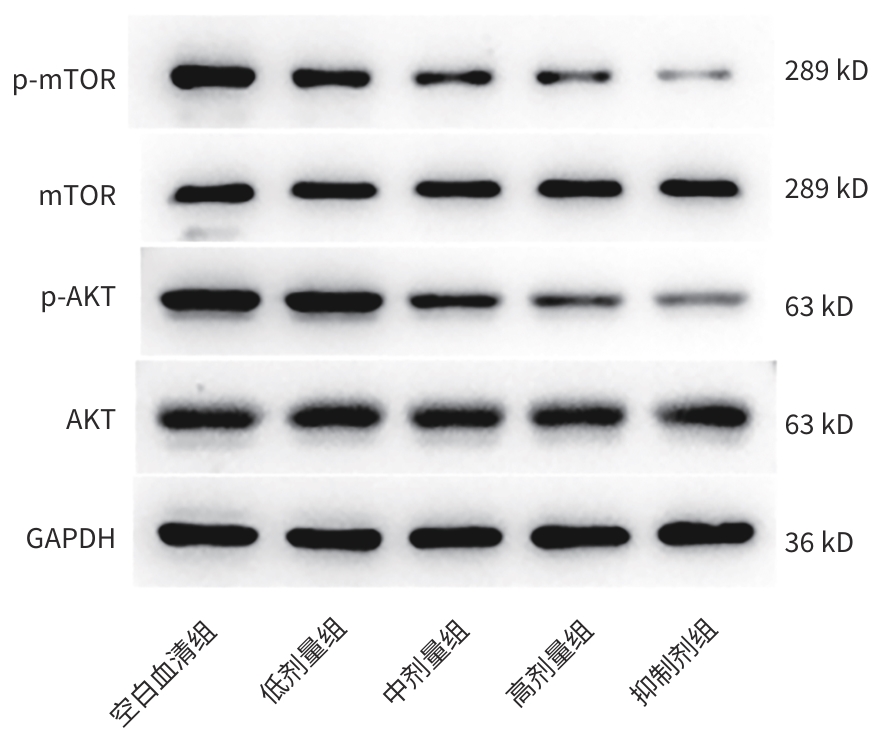
| [1] |
WANG J, ZHANG CL, TANG KY, et al. Research advances in traditional Chinese medicine in regulating epithelial-mesenchymal transformation to inhibit hepatocellular carcinoma metastasis[J]. J Clin Hepatol, 2022, 38( 11): 2636- 2642. DOI: 10.3969/j.issn.1001-5256.2022.11.040. |
| [2] |
SEVER R, BRUGGE JS. Signal transduction in cancer[J]. Cold Spring Harb Perspect Med, 2015, 5( 4): a006098. DOI: 10.1101/cshperspect.a006098. |
| [3] |
LEE G, ZHENG YX, CHO S, et al. Post-transcriptional regulation of de novo lipogenesis by mTORC1-S6K1-SRPK2 signaling[J]. Cell, 2017, 171( 7): 1545- 1558. e 18. DOI: 10.1016/j.cell.2017.10.037. |
| [4] |
PATRA T, MEYER K, RAY RB, et al. A combination of AZD5363 and FH5363 induces lethal autophagy in transformed hepatocytes[J]. Cell Death Dis, 2020, 11( 7): 540. DOI: 10.1038/s41419-020-02741-1. |
| [5] |
WANG HY, CHEN L. Tumor microenviroment and hepatocellular carcinoma metastasis[J]. J Gastroenterol Hepatol, 2013, 28( Suppl 1): 43- 48. DOI: 10.1111/jgh.12091. |
| [6] |
SUN C, LI B, LI L, et al. Effect of Huaier granules on patients with middle and advanced liver cancer[J]. J Changchun Univ Chin Med, 2022, 38( 10): 1130- 1133. DOI: 10.13463/j.cnki.cczyy.2022.10.016. |
| [7] |
|
| [8] |
WU MS, LIU H, TAN NH, et al. The effect of Biejiajian Pills on regulating the EGFR/MAPK/ERK pathway in MHCC-97H liver cancer cells[J]. J Beijing Univ Tradit Chin Med, 2024, 47( 3): 394- 406.
伍梦思, 刘华, 谭年花, 等. 鳖甲煎丸调控EGFR/MAPK/ERK通路对MHCC-97H肝癌细胞的影响[J]. 北京中医药大学学报, 2024, 47( 3): 394- 406.
|
| [9] |
ZHONG XD, WEN B, SUN HT, et al. Mechanism of Biejiajian Wan against EMT of hepatocellular carcinoma cells through NF-κB signaling pathway[J]. Chin J Exp Tradit Med Formulae, 2022, 28( 1): 24- 32. DOI: 10.13422/j.cnki.syfjx.20212421. |
| [10] |
HUANG JJ, HUANG HN, ZHANG WF, et al. Bie Jia Jian pill combined with bone mesenchymal stem cells regulates microRNA-140 to suppress hepatocellular carcinoma stem cells[J]. Int J Stem Cells, 2021, 14( 3): 275- 285. DOI: 10.15283/ijsc20157. |
| [11] |
SHAO FL, CHEN QP, BI Q, et al. Intervention mechanism of Biejiajian Wan on primary liver cancer by regulating lncRNA SNHG5/miRNA-26a-5p/GSK-3β signal axis[J]. Chin J Exp Tradit Med Formulae, 2024, 30( 4): 107- 113. DOI: 10.13422/j.cnki.syfjx.20230730. |
| [12] |
TAO ZD, WU QY, LAN XH, et al. Effect of physcion on proliferation and glycolysis of liver cancer cells and its mechanism[J]. Chin Clin Oncol, 2022, 27( 9): 769- 775. DOI: 10.3969/j.issn.1009-0460.2022.09.001. |
| [13] |
ZANOTELLI MR, ZHANG J, REINHART-KING CA. Mechanoresponsive metabolism in cancer cell migration and metastasis[J]. Cell Metab, 2021, 33( 7): 1307- 1321. DOI: 10.1016/j.cmet.2021.04.002. |
| [14] |
NIU YQ, LIU F, WANG XY, et al. miR-183-5p promotes HCC migration/invasion via increasing aerobic glycolysis[J]. Onco Targets Ther, 2021, 14: 3649- 3658. DOI: 10.2147/OTT.S304117. |
| [15] |
WEI YH, YANG CX, YANG GM, et al. Inhibitory effect of downregulating HMGB2 expression on epithelial-mesenchymal transition of liver cancer LM3 cells and its AKT/mTOR signaling pathway mechanism[J]. J Jilin Univ Med Ed, 2024, 50( 1): 143- 149. DOI: 10.13481/j.1671-587X.20240118. |
| [16] |
DI SC, GONG M, LV JM, et al. Glycolysis-related biomarker TCIRG1 participates in regulation of renal cell carcinoma progression and tumor immune microenvironment by affecting aerobic glycolysis and AKT/mTOR signaling pathway[J]. Cancer Cell Int, 2023, 23( 1): 186. DOI: 10.1186/s12935-023-03019-0. |
| [17] |
YU FY, ZHAO XY, LI MT, et al. SLITRK6 promotes the progression of lung adenocarcinoma by regulating PI3K/AKT/mTOR signaling and Warburg effect[J]. Apoptosis, 2023, 28( 7-8): 1216- 1225. DOI: 10.1007/s10495-023-01838-0. |
| [18] |
SHI Z, ZHOU LY, ZHAO GD, et al. Effect of micro-ribonucleic acid-21 on the malignant biological behavior of cholangiocarcinoma cells by targeting the PTEN/PI3K/Akt pathway[J]. J Clin Hepatol, 2022, 38( 9): 2091- 2098. DOI: 10.3969/j.issn.1001-5256.2022.09.026. |
| [19] |
LI ZB, YE JX, WU Y, et al. Study on the mechanism of bezafibrate inhibiting glycolysis in lung adenocarcinoma through AKT/mTOR pathway[J]. J Guangdong Pharm Univ, 2024, 40( 1): 101- 104. DOI: 10.16809/j.cnki.2096-3653.2023030703. |
| [20] |
QU YQ, ZHANG QY, TAN XY, et al. Effect of nuciferine against the proliferation of cholangiocarcinoma cells through Akt/mTOR/4EBP1-glycolytic pathway[J]. Nat Prod Res Dev, 2023, 35( 8): 1297- 1304, 1379. DOI: 10.16333/j.1001-6880.2023.8.002. |
| [21] |
DENG DJ, LI L, TAN XY, et al. Effect and mechanism of icaritin on inhibiting proliferation of intrahepatic cholangiocarcinoma cells by Akt/mTOR-mediated glycolysis[J]. Chin Tradit Herb Drugs, 2022, 53( 10): 3061- 3069. DOI: 10.7501/j.issn.0253-2670.2022.10.016. |








 DownLoad:
DownLoad: