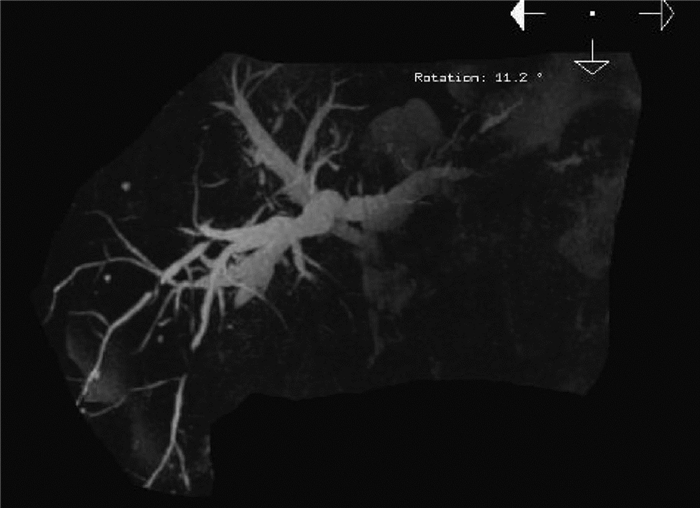
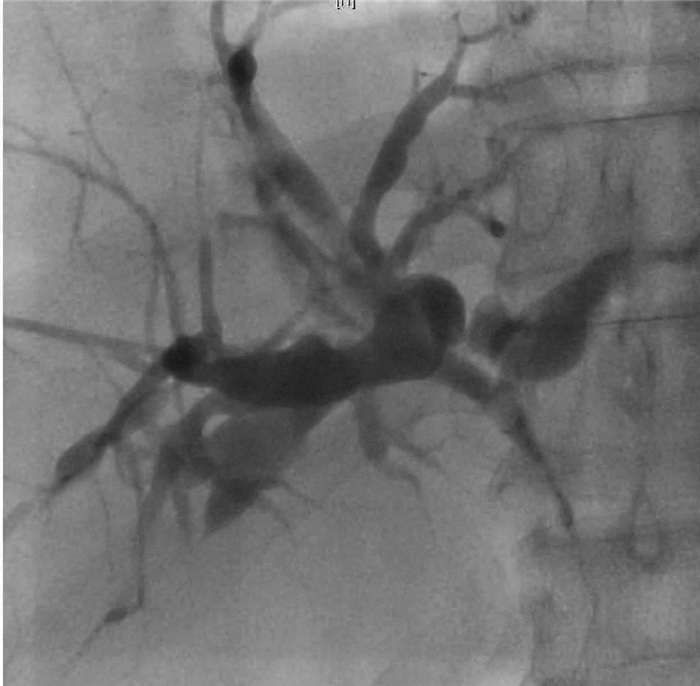
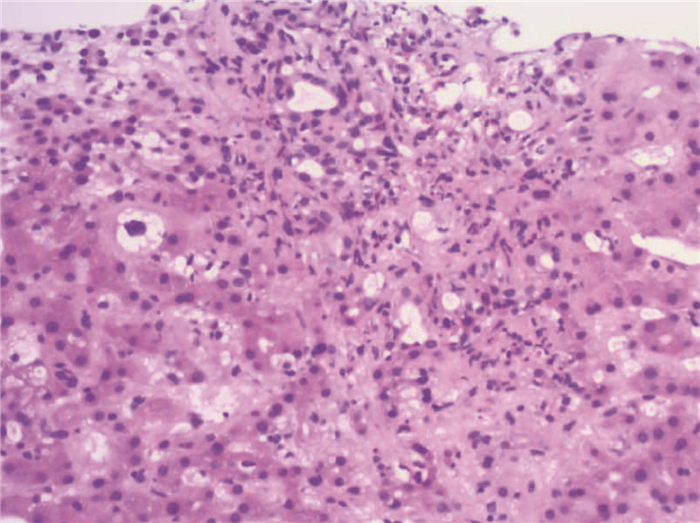
| [1] |
Bureau of Medical Administration, National Health Commission of the People's Republic of China. Guidelines for diagnosis and treatment of primary liver cancer in China (2019 edition)[J]. J Clin Hepatol, 2020, 36(2): 277-292. (in Chinese) DOI: 10.3969/j.issn.1001-5256.2020.02.007 |
| [2] |
MINAMI Y, KUDO M. Hepatocellular carcinoma with obstructive jaundice: Endoscopic and percutaneous biliary drainage[J]. Dig Dis, 2012, 30(6): 592-597. DOI: 10.1159/000343087 |
| [3] |
YU H, GUO Z, XING W, et al. Bile culture and susceptibility testing of malignant biliary obstruction via PTBD[J]. Cardiovasc Intervent Radiol, 2012, 35(5): 1136-1144. DOI: 10.1007/s00270-011-0263-2 |
| [4] |
KUDO M, FINN RS, QIN S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: A randomised phase 3 non-inferiority trial[J]. Lancet, 2018, 391(10126): 1163-1173. DOI: 10.1016/S0140-6736(18)30207-1 |
| [5] |
Chinese Society of Clinical Oncology Guidance Working Committee. Guidelines of Chinese Society of Clinical Oncology (CSCO) management of immune checkpoint inhibitor-related toxicity[M]. Beijing: People's Medical Publishing House, 2019: 33-36. (in Chinese)
中国临床肿瘤学会指南工作委员会. 中国临床肿瘤学会(CSCO)免疫检查点抑制剂相关的毒性管理指南2019[M]. 北京:人民卫生出版社, 2019: 33-36.
|
| [6] |
|
| [7] |
ZHANG QY, CHEN LL, GAO F, et al. Pathological features of immune-mediated hepatitis due to immune checkpoint inhibitors and anti-angiogenesis targeted therapy[J]. Chin J Pathol, 2020, 49(4): 329-335. (in Chinese) http://d.wanfangdata.com.cn/periodical/zhblx202004005 |
| [8] |
TROTTI A, BYHARDT R, STETZ J, et al. Common toxicity criteria: Version 2.0. an improved reference for grading the acute effects of cancer treatment: Impact on radiotherapy[J]. Int J Radiat Oncol Biol Phys, 2000, 47(1): 13-47. DOI: 10.1016/S0360-3016(99)00559-3 |
| [9] |
REED GB Jr, COX AJ Jr. The human liver after radiation injury. A form of veno-occlusive disease[J]. Am J Pathol, 1966, 48(4): 597-611.
|
| [10] |
WU DC. Radioactive medicine[M]. Beijing: Military Medical Science Press, 2001: 104-105. (in Chinese)
吴德昌. 放射医学[M]. 北京:军事医学科学出版社, 2001: 104-105.
|



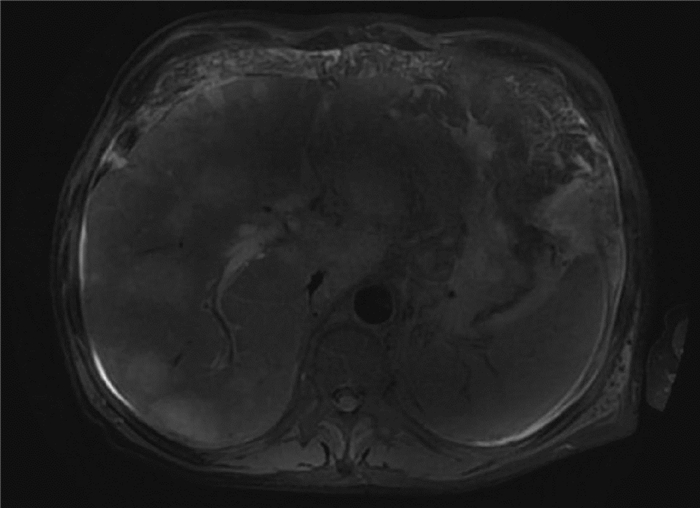




 DownLoad:
DownLoad: