| [1] |
Bureau of Medical Administration, National Health Commission of the People's Republic of China. Guidelines for diagnosis and treatment of primary liver cancer in China (2019 edition)[J]. J Clin Hepatol, 2020, 36(2): 277-292. (in Chinese) DOI: 10.3969/j.issn.1001-5256.2020.02.007 |
| [2] |
PARDOLL DM. The blockade of immune checkpoints in cancer immunotherapy[J]. Nat Rev Cancer, 2012, 12(4): 252-264. DOI: 10.1038/nrc3239 |
| [3] |
EL-KHOUEIRY AB, SANGRO B, YAU T, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): An open-label, non-comparative, phase 1/2 dose escalation and expansion trial[J]. Lancet, 2017, 389(10088): 2492-2502. DOI: 10.1016/S0140-6736(17)31046-2 |
| [4] |
CROCENZI TS, EL-KHOUEIRY AB, YAU T, et al. Nivolumab in sorafenib-naive and-experienced patients with advanced hepatocellular carcinoma: CheckMate 040 study[J]. J Clin Oncol, 2017, 35(15 Suppl): 4013.
|
| [5] |
YAU T, HSU C, KIM TY, et al. Nivolumab in advanced hepatocellular carcinoma: Sorafenib-experienced Asian cohort analysis[J]. J Hepatol, 2019, 71(3): 543-552. DOI: 10.1016/j.jhep.2019.05.014 |
| [6] |
KUDO M, MATILLA A, SANTORO A, et al. Checkmate-040: Nivolumab (NIVO) in patients (pts) with advanced hepatocellular carcinoma (aHCC) and Child-Pugh B (CPB) status[J]. J Clin Oncol, 2019, 37(4 Suppl): 327.
|
| [7] |
KAMBHAMPATI S, BAUER KE, BRACCI PM, et al. Nivolumab in patients with advanced hepatocellular carcinoma and Child-Pugh class B cirrhosis: Safety and clinical outcomes in a retrospective case series[J]. Cancer, 2019, 125(18): 3234-3241. DOI: 10.1002/cncr.32206 |
| [8] |
YAU T, PARK JW, FINN RS, et al. CheckMate 459: A randomized, multi-center phase Ⅲ study of nivolumab (NIVO) vs sorafenib (SOR) as first-line (1L) treatment in patients (pts) with advanced hepatocellular carcinoma (aHCC)[J]. Ann Oncol, 2019, 30(Suppl 5): v874-v875.
|
| [9] |
SUN YK. Interpretation of CSCO guidelines for the diagnosis and treatment of primary liver cancer: Systemic treatment[J/CD]. Electronic J Liver Tumor, 2018, 5(3): 11-14. (in Chinese)
孙永琨. 2018《CSCO原发性肝癌诊疗指南》解读——全身治疗部分[J/CD]. 肝癌电子杂志, 2018, 5(3): 11-14.
|
| [10] |
|
| [11] |
ZHU AX, FINN RS, EDELINE J, et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): A non-randomised, open-label phase 2 trial[J]. Lancet Oncol, 2018, 19(7): 940-952. DOI: 10.1016/S1470-2045(18)30351-6 |
| [12] |
KUDO M, FINN RS, EDELINE J, et al. Updated efficacy and safety of KEYNOTE-224: A phase Ⅱ study of pembrolizumab (pembro) in patients with advanced hepatocellular carcinoma (HCC)[J]. J Clin Oncol, 2020, 38(4 Suppl): 518.
|
| [13] |
FINN RS, RYOO BY, MERLE P, et al. Pembrolizumab as second-line therapy in patients with advanced hepatocellular carcinoma in KEYNOTE-240: A randomized, double-blind, phase Ⅲ trial[J]. J Clin Oncol, 2020, 38(3): 193-202. DOI: 10.1200/JCO.19.01307 |
| [14] |
BENSON AB, D'ANGELICA MI, ABBOTT DE, et al. Guidelines insights: Hepatobiliary cancers, Version 2.2019[J]. J Natl Compr Canc Netw, 2019, 17(4): 302-310. DOI: 10.6004/jnccn.2019.0019 |
| [15] |
QIN S, REN Z, MENG Z, et al. Camrelizumab in patients with previously treated advanced hepatocellular carcinoma: A multicentre, open-label, parallel-group, randomised, phase 2 trial[J]. Lancet Oncol, 2020, 21(4): 571-580. DOI: 10.1016/S1470-2045(20)30011-5 |
| [16] |
WAINBERG ZA, SEGAL NH, JAEGER D, et al. Safety and clinical activity of durvalumab monotherapy in patients with hepatocellular carcinoma (HCC)[J]. J Clin Oncol, 2017, 35(15 Suppl): 4071.
|
| [17] |
FENG Z, RONG P, WANG W. Meta-analysis of the efficacy and safety of PD-1/PD-L1 inhibitors administered alone or in combination with anti-VEGF agents in advanced hepatocellular carcinoma[J]. Gut, 2020, 69(10): 1904-1906. DOI: 10.1136/gutjnl-2019-320116 |
| [18] |
KUDO M. Immuno-oncology therapy for hepatocellular carcinoma: Current status and ongoing trials[J]. Liver Cancer, 2019, 8(4): 221-238. DOI: 10.1159/000501501 |
| [19] |
ZHU AX, FINN RS, IKEDA M, et al. A phase Ib study of lenvatinib (LEN) plus pembrolizumab (PEMBRO) in unresectable hepatocellular carcinoma (uHCC)[J]. J Clin Oncol, 2020, 38(15 Suppl): 4519.
|
| [20] |
LLOVET JM, KUDO M, CHENG AL, et al. Lenvatinib (len) plus pembrolizumab (pembro) for the first-line treatment of patients (pts) with advanced hepatocellular carcinoma (HCC): Phase 3 LEAP-002 study[J]. J Clini Oncol, 2019, 37(15 Suppl): TPS4152.
|
| [21] |
KUDO M, IKEDA M, MOTOMURA K, et al. A phase Ib study of lenvatinib (LEN) plus nivolumab (NIV) in patients (pts) with unresectable hepatocellular carcinoma (uHCC): Study 117[J]. J Clin Oncol, 2020, 38(4 Suppl): 513.
|
| [22] |
XU J, ZHANG Y, JIA R, et al. Anti-PD-1 antibody SHR-1210 combined with apatinib for advanced hepatocellular carcinoma, gastric, or esophagogastric junction cancer: An open-label, dose escalation and expansion study[J]. Clin Cancer Res, 2019, 25(2): 515-523. DOI: 10.1158/1078-0432.CCR-18-2484 |
| [23] |
KUDO M, MOTOMURA K, WADA Y, et al. First-line avelumab + axitinib in patients with advanced hepatocellular carcinoma: Results from a phase 1b trial (VEGF Liver 100)[J]. J Clin Oncol, 2019, 37(15 Suppl): 4072.
|
| [24] |
RIMASSA L, CHENG AL, BRAITEH F, et al. Phase Ⅲ (COSMIC-312) study of cabozantinib (C) in combination with atezolizumab (A) vs sorafenib (S) in patients (pts) with advanced hepatocellular carcinoma (aHCC) who have not received previous systemic anticancer therapy[J]. Ann Oncol, 2019, 30(Suppl 5): v320.
|
| [25] |
LEE M, RYOO BY, HSU CH, et al. Randomised efficacy and safety results for atezolizumab (Atezo) + bevacizumab (Bev) in patients (pts) with previously untreated, unresectable hepatocellular carcinoma (HCC)[J]. Ann Oncol, 2019, 30(Suppl 5): v875.
|
| [26] |
FINN RS, QIN S, IKEDA M, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma[J]. N Engl J Med, 2020, 382(20): 1894-1905. DOI: 10.1056/NEJMoa1915745 |
| [27] |
CHENG AL, QIN S, IKEDA M, et al. IMbrave150: Efficacy and safety results from a ph Ⅲ study evaluating atezolizumab (atezo)+bevacizumab (bev) vs sorafenib (Sor) as first treatment (tx) for patients (pts) with unresectable hepatocellular carcinoma (HCC)[J]. Ann Oncol, 2019, 30(Suppl 9): ix186-ix187.
|
| [28] |
|
| [29] |
YAU T, KANG YK, KIM TY, et al. Nivolumab (NIVO) + ipilimumab (IPI) combination therapy in patients (pts) with advanced hepatocellular carcinoma (aHCC): Results from CheckMate 040[J]. J Clin Oncol, 2019, 37(15 Suppl): 4012.
|
| [30] |
HE AR, YAU T, HSU C, et al. Nivolumab (NIVO) + ipilimumab (IPI) combination therapy in patients (pts) with advanced hepatocellular carcinoma (aHCC): Subgroup analyses from CheckMate 040[J]. J Clin Oncol, 2020, 38(4 Suppl): 512.
|
| [31] |
KELLEY RK, SANGRO B, HARRIS WP, et al. Efficacy, tolerability, and biologic activity of a novel regimen of tremelimumab (T) in combination with durvalumab (D) for patients (pts) with advanced hepatocellular carcinoma (aHCC)[J]. J Clin Oncol, 2020, 38(15 Suppl): 4508.
|
| [32] |
ABOU-ALFA GK, CHAN SL, FURUSE J, et al. A randomized, multicenter phase 3 study of durvalumab (D) and tremelimumab (T) as first-line treatment in patients with unresectable hepatocellular carcinoma (HCC): HIMALAYA study[J]. J Clin Oncol, 2018, 36(15 Suppl): TPS4144.
|
| [33] |
QIN S, CHEN Z, LIU Y, et al. A phase Ⅱ study of anti-PD-1 antibody camrelizumab plus FOLFOX4 or GEMOX systemic chemotherapy as first-line therapy for advanced hepatocellular carcinoma or biliary tract cancer[J]. J Clin Oncol, 2019, 37(15 Suppl): 4074.
|
| [34] |
YAU T, ZAGONEL V, SANTORO A, et al. Nivolumab (NIVO)+ipilimumab (IPI)+cabozantinib (CABO) combination therapy in patients (pts) with advanced hepatocellular carcinoma (aHCC): Results from CheckMate 040[J]. J Clin Oncol, 2020, 38(4 Suppl): 478.
|



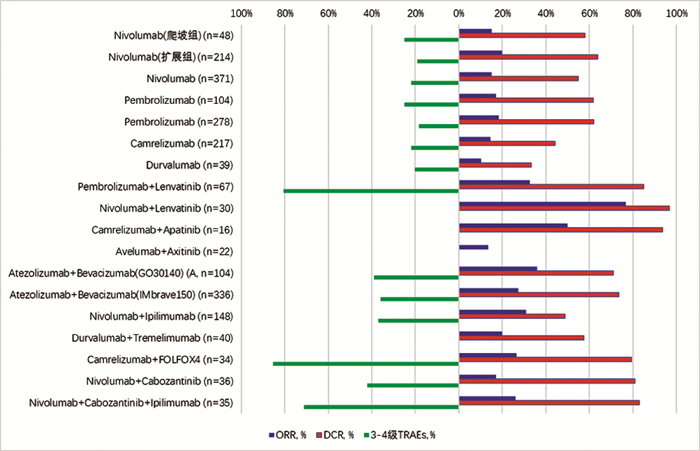




 DownLoad:
DownLoad: