| [1] |
Polaris Observatory Collaborators. Global prevalence, treatment, and prevention of hepatitis B virus infection in 2016: A modelling study[J]. Lancet Gastroenterol Hepatol, 2018, 3(6): 383-403. DOI: 10.1016/S2468-1253(18)30056-6. |
| [2] |
Chinese Society of Infectious Diseases, Chinese Medical Association; Chinese Society of Hepatology, Chinese Medical Association. Guidelines for the prevention and treatment of chronic hepatitis B (version 2019)[J]. J Clin Hepatol, 2019, 35(12): 2648-2669. DOI: 10.3969/j.issn.1001-5256.2019.12.007. |
| [3] |
SARIN SK, KUMAR M, LAU GK, et al. Asian-Pacific clinical practice guidelines on the management of hepatitis B: A 2015 update[J]. Hepatol Int, 2016, 10(1): 1-98. DOI: 10.1007/s12072-015-9675-4. |
| [4] |
TERRAULT NA, LOK A, MCMAHON BJ, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance[J]. Hepatology, 2018, 67(4): 1560-1599. DOI: 10.1002/hep.29800. |
| [5] |
LOK AS, MCMAHON BJ, BROWN RS Jr, et al. Antiviral therapy for chronic hepatitis B viral infection in adults: A systematic review and meta-analysis[J]. Hepatology, 2016, 63(1): 284-306. DOI: 10.1002/hep.28280. |
| [6] |
European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection[J]. J Hepatol, 2017, 67(2): 370-398.
|
| [7] |
BERTOLETTI A, KENNEDY PT. The immune tolerant phase of chronic HBV infection: New perspectives on an old concept[J]. Cell Mol Immunol, 2015, 12(3): 258-263. DOI: 10.1038/cmi.2014.79. |
| [8] |
KOFFAS A, PETERSEN J, KENNEDY PT. Reasons to consider early treatment in chronic hepatitis B patients[J]. Antiviral Res, 2020, 177: 104783. DOI: 10.1016/j.antiviral.2020.104783. |
| [9] |
WONG GL. Management of chronic hepatitis B patients in immunetolerant phase: What latest guidelines recommend[J]. Clin Mol Hepatol, 2018, 24(2): 108-113. DOI: 10.3350/cmh.2017.0068. |
| [10] |
KIM GA, LIM YS, HAN S, et al. High risk of hepatocellular carcinoma and death in patients with immune-tolerant-phase chronic hepatitis B[J]. Gut, 2018, 67(5): 945-952. DOI: 10.1136/gutjnl-2017-314904. |
| [11] |
CHANG Y, CHOE WH, SINN DH, et al. Nucleos(t)ide analogue treatment for patients with hepatitis B virus (HBV) e antigen-positive chronic HBV genotype C infection: A nationwide, multicenter, retrospective study[J]. J Infect Dis, 2017, 216(11): 1407-1414. DOI: 10.1093/infdis/jix506. |
| [12] |
WONG VW, HUI AJ, WONG GL, et al. Four-year outcomes after cessation of tenofovir in immune-tolerant chronic hepatitis B patients[J]. J Clin Gastroenterol, 2018, 52(4): 347-352. DOI: 10.1097/MCG.0000000000000852. |
| [13] |
FELD JJ, TERRAULT NA, LIN HS, et al. Entecavir and Peginterferon Alfa-2a in adults with hepatitis B e antigen-positive immune-tolerant chronic hepatitis B virus infection[J]. Hepatology, 2019, 69(6): 2338-2348. DOI: 10.1002/hep.30417. |
| [14] |
MOHER D, LIBERATI A, TETZLAFF J, et al. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement[J]. BMJ, 2009, 339: b2535. DOI: 10.1136/bmj.b2535. |
| [15] |
HIGGINS JP, ALTMAN DG, GØTZSCHE PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials[J]. BMJ, 2011, 343: d5928. DOI: 10.1136/bmj.d5928. |
| [16] |
WELLS G, SHEA B, O'CONNELL D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2013.
|
| [17] |
ARTAN R. Lamivudine monotherapy in children with immune-tolerant chronic hepatitis B virus[J]. J Chemother, 2005, 17(2): 198-202. DOI: 10.1179/joc.2005.17.2.198. |
| [18] |
CHAN HL, CHAN CK, HUI AJ, et al. Effects of tenofovir disoproxil fumarate in hepatitis B e antigen-positive patients with normal levels of alanine aminotransferase and high levels of hepatitis B virus DNA[J]. Gastroenterology, 2014, 146(5): 1240-1248. DOI: 10.1053/j.gastro.2014.01.044. |
| [19] |
LAU GK, NANJI A, HOU J, et al. Thymosin-alpha1 and famciclovir combination therapy activates T-cell response in patients with chronic hepatitis B virus infection in immune-tolerant phase[J]. J Viral Hepat, 2002, 9(4): 280-287. DOI: 10.1046/j.1365-2893.2002.00361.x. |
| [20] |
LEUNG NW, HERRMANN E, LAU GK, et al. Early viral kinetics with telbivudine, tenofovir or combination of both in immunotolerant patients with hepatitis B e antigen-positive chronic hepatitis B[J]. Infect Dis Ther, 2014, 3(2): 191-202. DOI: 10.1007/s40121-014-0039-5. |
| [21] |
PODDAR U, YACHHA SK, AGARWAL J, et al. Cure for immune-tolerant hepatitis B in children: Is it an achievable target with sequential combo therapy with lamivudine and interferon?[J]. J Viral Hepat, 2013, 20(5): 311-316. DOI: 10.1111/jvh.12007. |
| [22] |
WU ZX, CHEN FS, ZHOU XL, et al. Tenofovir and telbivudine combination therapy rapidly decreases viral loads in immune-tolerant chronic hepatitis B patients awaiting assisted reproduction: An open-label, randomized, controlled study[J]. Eur J Gastroenterol Hepatol, 2019, 31(7): 832-835. DOI: 10.1097/MEG.0000000000001345. |
| [23] |
ZHU S, ZHANG H, DONG Y, et al. Antiviral therapy in hepatitis B virus-infected children with immune-tolerant characteristics: A pilot open-label randomized study[J]. J Hepatol, 2018, 68(6): 1123-1128. DOI: 10.1016/j.jhep.2018.01.037. |
| [24] |
KAN YT, GAN JH, SUN W, et al. Effect of polyethylene glycol (peg) interferon therapy on Th17/Treg in patients with chronic hepatitis B[J]. Progr Mod Biomed, 2016, 16(13): 2490-2492. DOI: 10.13241/j.cnki.pmb.2016.13.023. |
| [25] |
ZHU Y. Analysis of the correlation between the change of chronic hepatitis B cytokines and the therapeutic effect of interferon[D]. Urumqi: Xinjiang Medical University, 2020.
朱艳芳. 慢乙肝细胞因子的变化与干扰素治疗疗效的相关性分析[D]. 乌鲁木齐: 新疆医科大学, 2020.
|
| [26] |
LEE HA, LEE HW, KIM IH, et al. Extremely low risk of hepatocellular carcinoma development in patients with chronic hepatitis B in immune-tolerant phase[J]. Aliment Pharmacol Ther, 2020, 52(1): 196-204. DOI: 10.1111/apt.15741. |
| [27] |
LEE HW, KIM EH, LEE J, et al. Correction to: Natural history of untreated hbeag-positive chronic HBV infection with persistently elevated HBV DNA but normal alanine aminotransferase[J]. Clin Transl Gastroenterol, 2020, 11(5): e00183. DOI: 10.14309/ctg.0000000000000183. |
| [28] |
GU Y, LIAN Y, ZHENG Q, et al. Association among cytokine profiles of innate and adaptive immune responses and clinical-virological features in untreated patients with chronic hepatitis B[J]. BMC Infect Dis, 2020, 20(1): 509. DOI: 10.1186/s12879-020-05233-x. |
| [29] |
LI MH, CHEN QQ, ZHANG L, et al. Association of cytokines with hepatitis B virus and its antigen[J]. J Med Virol, 2020. [Online ahead of print] DOI: 10.1002/jmv.26301. |
| [30] |
ZHAO JH, LI JF, MAO XR. Effect of T-lymphocyte phenotype on immune status in chronic hepatitis B virus infection and its application value[J]. J Clin Hepatol, 2020, 36(5): 1019-1023. DOI: 10.3969/j.issn.1001-5256.2020.05.014. |
| [31] |
JENG WJ, LOK AS. Should treatment indications for chronic hepatitis B be expanded?[J]. Clin Gastroenterol Hepatol, 2020. DOI: 10.1016/j.cgh.2020.04.091. |



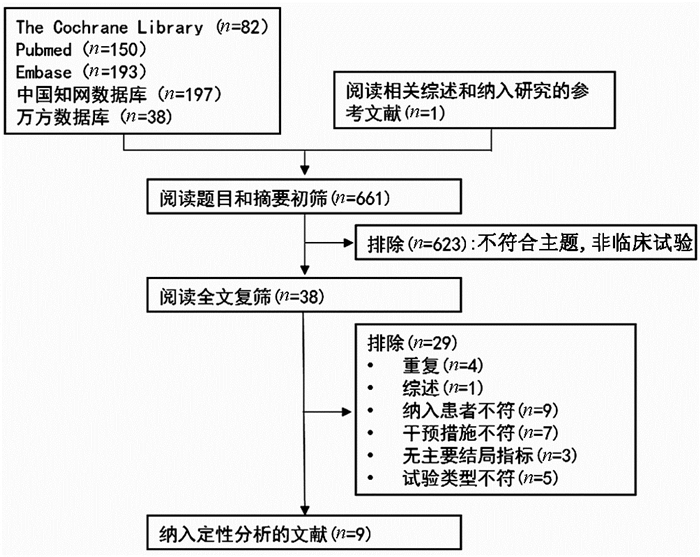




 DownLoad:
DownLoad: