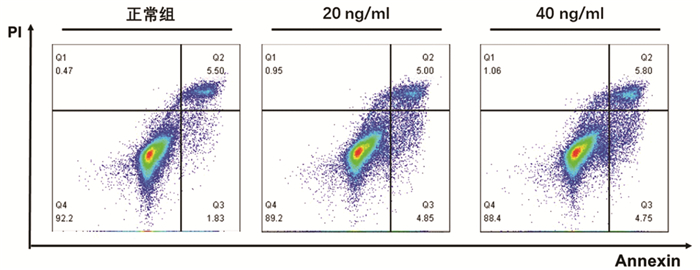
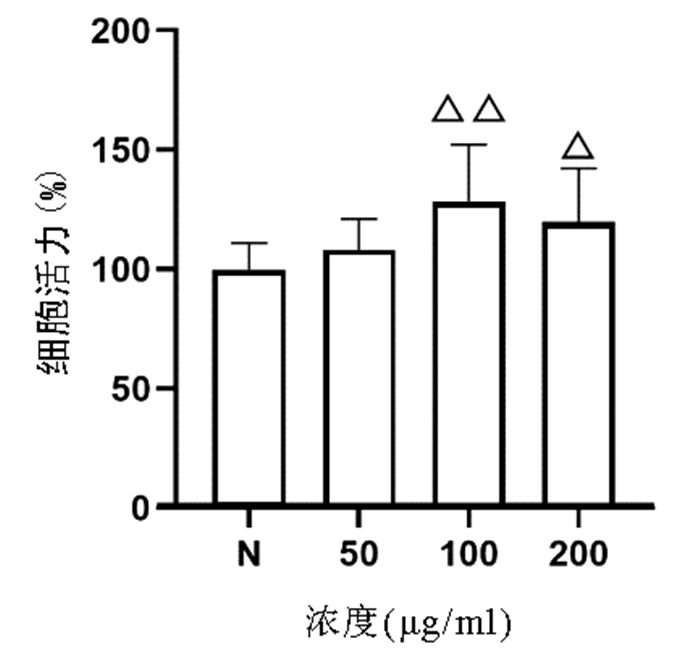
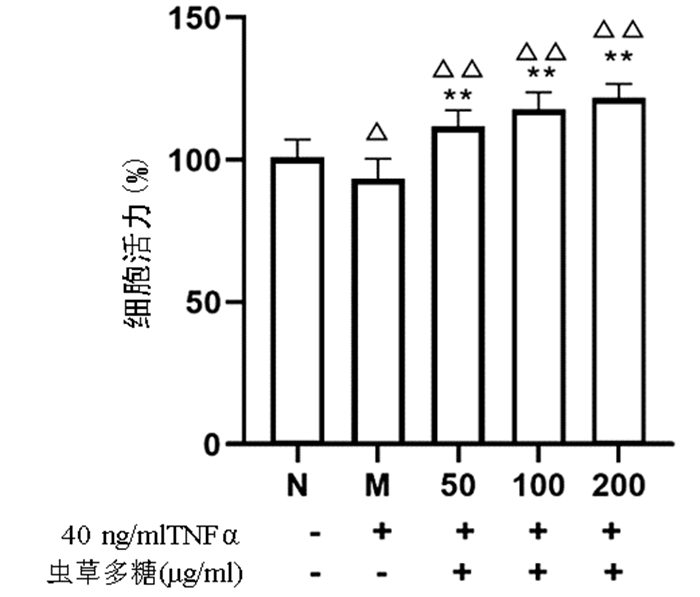
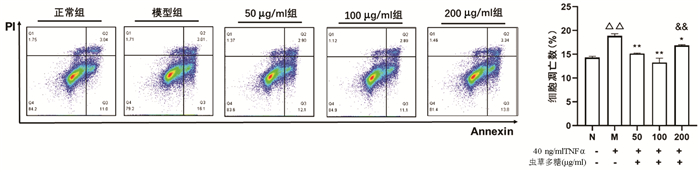
| [1] | Gong Jing, Jie XinKe. Effect of endoplasmic reticulum stress and autophagy on hepatocyte apoptosis[J]. Journal of Clinical Hepatology, 2019, 35(12): 2828-2832. doi: 10.3969/j.issn.1001-5256.2019.12.041 |
| [2] | Gao Si, Zhao XiangXuan, Lu ZaiMing. Role of cell apoptosis regulated by P53 in treatment of hepatocellular carcinoma[J]. Journal of Clinical Hepatology, 2017, 33(7): 1373-1376. doi: 10.3969/j.issn.1001-5256.2017.07.038 |
| [3] | Zhang ChaoYa, Zhao XiangXuan, Lu ZaiMing, Guo QiYong. Research advances in sorafenib- induced apoptotic signaling pathways in liver cancer cells[J]. Journal of Clinical Hepatology, 2016, 32(4): 816-820. doi: 10.3969/j.issn.1001-5256.2016.04.048 |
| [4] | Pan LiuLan, Jia ShengNan, Ma JingTing, Tai JingHua, Jin ZhenJing. Effect of decoy receptor 3 gene on hepatocyte apoptosis[J]. Journal of Clinical Hepatology, 2016, 32(7): 1330-1333. doi: 10.3969/j.issn.1001-5256.2016.07.023 |
| [5] | Zhang Xu, Wang Yu, Ma Juan, Wang ZhenZhen, Yuan Juan, Ma Yan, Liu Xin. Role of endoplasmic reticulum stress-mediated apoptosis in liver fibrosis[J]. Journal of Clinical Hepatology, 2015, 31(9): 1537-1539. doi: 10.3969/j.issn.1001-5256.2015.09.044 |
| [6] | Wang YingChun, Kong WeiZong, Jin QingMei, Chen Juan. Effect of salvianolic acid B on hepatocyte apoptosis in rats with nonalcoholic steatohepatitis[J]. Journal of Clinical Hepatology, 2014, 30(12): 1325-1329. doi: 10.3969/j.issn.1001-5256.2014.12.022 |
| [7] | Wang XiXun, Jiang LiXin, Wang JingLin, Liang Jing, Zhai HuiYuan, Xia XianMing. Experimental study on hepatocyte apoptosis induced by acute biliary tract infection[J]. Journal of Clinical Hepatology, 2014, 30(11): 1164-1168. doi: 10.3969/j.issn.1001-5256.2014.11.018 |
| [8] | Pan YuanWei, Ping Jian, Xu LieMing. Research advances in main signaling pathways for inducing apoptosis of activated hepatic stellate cells[J]. Journal of Clinical Hepatology, 2014, 30(2): 182-185. doi: 10.3969/j.issn.1001-5256.2014.02.021 |
| [9] | Xu Li, Ma KeXin, Dong Bing, Liu HaiWang, Liu QinLong, Gao ZhenMing, Sun DeGuang, Zhang RiXin, Wang HaiBo, Wang LiMing, Liang Rui. Regulatory effect of miRNAs on apoptosis, migration, and cell cycle of hepatocellular carcinoma cells[J]. Journal of Clinical Hepatology, 2013, 29(7): 554-557. |
| [10] | Tai BoJun, Yao Min, Gu Xing, Shi Yun, Yu DanDan, Chen Jie, Zheng WenJie, Yao DengFu, Lu ShaoLin. Effects of silencing glypican- 3 gene transcription on proliferation and apoptosis of hepatoma cells[J]. Journal of Clinical Hepatology, 2013, 29(11): 863-866. doi: 10.3969/j.issn.1001-5256.2013.10.015 |
| [11] | Weng ShanGeng, Xu ChangGuo, Sun Ying, Shi Zheng, Lin LiJuan. Study on the apoptosis of rat hepatic stellate cells induced by kapamycin[J]. Journal of Clinical Hepatology, 2012, 28(3): 219-222. |
| [12] | Qiao ChunPing, Chen YueXiang. Advances in study of the relationship between apoptosis and alcoholic liver disease[J]. Journal of Clinical Hepatology, 2012, 28(5): 386-390. |
| [13] | Sun YunTao, Ban Bo, Wang YiBo, Liu Yang. Effect of fenofibrate on hepatocyte apoptosis and hepatic glycogen in rats with obstructive jaundice[J]. Journal of Clinical Hepatology, 2011, 27(9): 947-950. |
| [14] | Gao Jie, Xu ChunHai, Liang Ming, Yu JianWu, Li ShuChen. Effect of fibronectin on protection of rat hepatic stellate cells HSC-T6 from TRAIL-induced apoptosis[J]. Journal of Clinical Hepatology, 2011, 27(11): 1199-1202. |
| [15] | Zhang LiLi, Guo XiaoHong, Liu LiXin, Zhang QianQian. IGFBPrP1 siRNA induced apoptosis in rat hepatic stellate cells and its mechanism[J]. Journal of Clinical Hepatology, 2011, 27(9): 965-969+979. |
| [17] | Zhang Biao, Sui Tao, Yu XiuYing, Cao YongXian, Yao Yuan, He Hong. Significance of detecting apoptosis of PBMC and cytokine of supernatant induced by PHA in chronic hepatitis B[J]. Journal of Clinical Hepatology, 2004, 20(3): 138-140. |
| [20] | Wang JunXue, Wang GuoJun, Cai Xiong. The effects of oxymatrine on apoptosis induced by TNF-α in human liver cell line L02[J]. Journal of Clinical Hepatology, 2001, 17(4): 210-211+217. |














 DownLoad:
DownLoad: