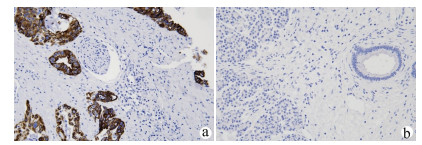
| [1] |
|
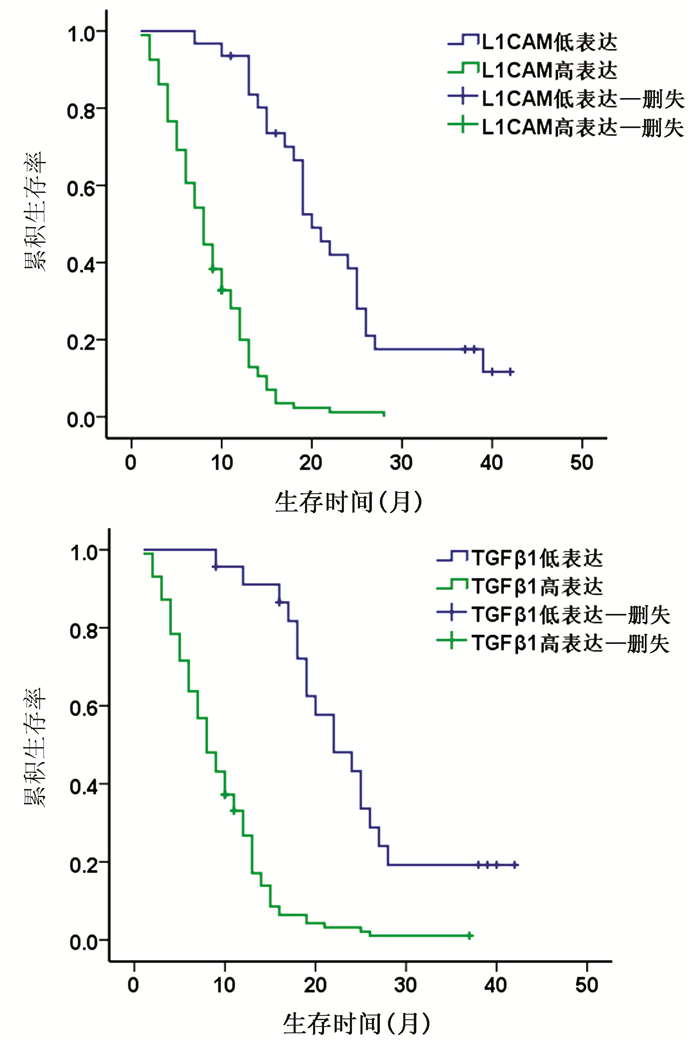
| [2] |
CHEN W, ZHENG R, BAADE PD, et al. Cancer statistics in China, 2015[J]. CA Cancer J Clin, 2016, 66(2): 115-132. DOI: 10.3322/caac.21338. |
| [3] |
SAMATA B, TAKAICHI R, ISHⅡ Y, et al. L1CAM is a marker for enriching corticospinal motor neurons in the developing brain[J]. Front Cell Neurosci, 2020, 14: 31. DOI: 10.3389/fncel.2020.00031. |
| [4] |
LINNEBERG C, TOFT C, KJAER-SORENSEN K, et al. L1cam-mediated developmental processes of the nervous system are differentially regulated by proteolytic processing[J]. Sci Rep, 2019, 9(1): 3716. DOI: 10.1038/s41598-019-39884-x. |
| [5] |
SCHÄFER MK, ALTEVOGT P. L1CAM malfunction in the nervous system and human carcinomas[J]. Cell Mol Life Sci, 2010, 67(14): 2425-2437. DOI: 10.1007/s00018-010-0339-1. |
| [6] |
ALTEVOGT P, DOBERSTEIN K, FOGEL M. L1CAM in human cancer[J]. Int J Cancer, 2016, 138(7): 1565-1576. DOI: 10.1002/ijc.29658. |
| [7] |
ANGIOLINI F, CAVALLARO U. The pleiotropic role of L1CAM in tumor vasculature[J]. Int J Mol Sci, 2017, 18(2): 254. DOI: 10.3390/ijms18020254. |
| [8] |
KIM KK, SHEPPARD D, CHAPMAN HA. TGF-β1 signaling and tissue fibrosis[J]. Cold Spring Harb Perspect Biol, 2018, 10(4): a022293. DOI: 10.1101/cshperspect.a022293. |
| [9] |
TANG J, GIFFORD CC, SAMARAKOON R, et al. Deregulation of negative controls on TGF-β1 signaling in tumor progression[J]. Cancers (Basel), 2018, 10(6): 159. DOI: 10.3390/cancers10060159. |
| [10] |
LIANG M, LIU XC, LIU T, et al. GLI-1 facilitates the EMT induced by TGF-β1 in gastric cancer[J]. Eur Rev Med Pharmacol Sci, 2018, 22(20): 6809-6815. DOI: 10.26355/eurrev_201810_16148. |
| [11] |
|
| [12] |
MOON JR, OH SJ, LEE CK, et al. TGF-β1 protects colon tumor cells from apoptosis through XAF1 suppression[J]. Int J Oncol, 2019, 54(6): 2117-2126. DOI: 10.3892/ijo.2019.4776. |
| [13] |
FEZZA M, MOUSSA M, AOUN R, et al. DKK1 promotes hepatocellular carcinoma inflammation, migration and invasion: Implication of TGF-β1[J]. PLoS One, 2019, 14(9): e0223252. DOI: 10.1371/journal.pone.0223252. |
| [14] |
MARTELOSSI CEBINELLI GC, PAIVA TRUGILO K, BADARÓ GARCIA S, et al. TGF-β1 functional polymorphisms: A review[J]. Eur Cytokine Netw, 2016, 27(4): 81-89. DOI: 10.1684/ecn.2016.0382. |
| [15] |
SAMATOV TR, WICKLEIN D, TONEVITSKY AG. L1CAM: Cell adhesion and more[J]. Prog Histochem Cytochem, 2016, 51(2): 25-32. DOI: 10.1016/j.proghi.2016.05.001. |
| [16] |
PACE KR, DUTT R, GALILEO DS. Exosomal L1CAM stimulates glioblastoma cell motility, proliferation, and invasiveness[J]. Int J Mol Sci, 2019, 20(16): 3982. DOI: 10.3390/ijms20163982. |
| [17] |
WACHOWIAK R, KRAUSE M, MAYER S, et al. Increased L1CAM (CD171) levels are associated with glioblastoma and metastatic brain tumors[J]. Medicine (Baltimore), 2018, 97(38): e12396. DOI: 10.1097/MD.0000000000012396. |
| [18] |
ABDEL AZIM S, SPRUNG S, MUTZ-DEHBALAIE I, et al. L1CAM and HER2 expression in early endometrioid uterine cancer[J]. Int J Gynecol Pathol, 2017, 36(4): 356-363. DOI: 10.1097/PGP.0000000000000338. |
| [19] |
GANESH K, BASNET H, KAYGUSUZ Y, et al. L1CAM defines the regenerative origin of metastasis-initiating cells in colorectal cancer[J]. Nat Cancer, 2020, 1(1): 28-45. DOI: 10.1038/s43018-019-0006-x. |
| [20] |
TAMPAKIS A, TAMPAKI EC, NONNI A, et al. L1CAM expression in colorectal cancer identifies a high-risk group of patients with dismal prognosis already in early-stage disease[J]. Acta Oncol, 2020, 59(1): 55-59. DOI: 10.1080/0284186X.2019.1667022. |
| [21] |
YU H, ZHOU P, LI D, et al. L1CAM-positive expression is associated with poorer survival outcomes in resected non-small cell lung cancer patients[J]. Int J Clin Exp Pathol, 2019, 12(7): 2665-2671. http://www.ncbi.nlm.nih.gov/pubmed/31934096 |
| [22] |
ALTEVOGT P, BEN-ZE'EV A, GAVERT N, et al. Recent insights into the role of L1CAM in cancer initiation and progression[J]. Int J Cancer, 2020, 147(12): 3292-3296. DOI: 10.1002/ijc.33177. |
| [23] |
GIORDANO M, CAVALLARO U. Different shades of L1CAM in the pathophysiology of cancer stem cells[J]. J Clin Med, 2020, 9(5): 1502. DOI: 10.3390/jcm9051502. |
| [24] |
ABDEL AZIM S, SPRUNG S, MUTZ-DEHBALAIE I, et al. L1CAM and HER2 Expression in Early Endometrioid Uterine Cancer[J]. Int J Gynecol Pathol, 2017, 36(4): 356-363. DOI: 10.1097/PGP.0000000000000338. |



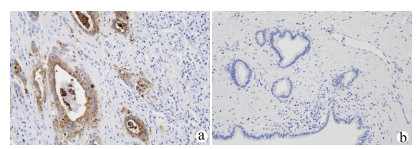




 DownLoad:
DownLoad: