| [1] |
WEDEMEYER H, DORE GJ, WARD JW. Estimates on HCV disease burden worldwide - filling the gaps[J]. J Viral Hepat, 2015, 22(Suppl 1): 1-5. DOI: 10.1111/jvh.12371. |
| [2] |
GONZALEZ HC, DUARTE-ROJO A. Virologic cure of hepatitis C: Impact on hepatic fibrosis and patient outcomes[J]. Curr Gastroenterol Rep, 2016, 18(7): 32. DOI: 10.1007/s11894-016-0508-y. |
| [3] |
LOUIE KS, MICALLEF JM, PIMENTA JM, et al. Prevalence of thrombocytopenia among patients with chronic hepatitis C: A systematic review[J]. J Viral Hepat, 2011, 18(1): 1-7. DOI: 10.1111/j.1365-2893.2010.01366.x. |
| [4] |
KEE KM, WANG JH, HUNG CH, et al. Improvement of thrombocytopenia in hepatitis C-related advanced fibrosis patients after sustained virological response[J]. Dig Dis Sci, 2013, 58(2): 556-561. DOI: 10.1007/s10620-012-2380-4. |
| [5] |
van der MEER AJ, MAAN R, VELDT BJ, et al. Improvement of platelets after SVR among patients with chronic HCV infection and advanced hepatic fibrosis[J]. J Gastroenterol Hepatol, 2016, 31(6): 1168-1176. DOI: 10.1111/jgh.13252. |
| [6] |
Chinese Society of Hepatology, Chinese Society of Infectious Diseases, Chinese Medical Association. The guideline of prevention and treatment for hepatitis C: A 2015 update[J]. J Clin Hepatol, 2015, 31(12): 1961-1979. DOI: 10.3969/j.issn.1001-5256.2015.12.003. |
| [7] |
European Association for the Study of the Liver. EASL recommendations on treatment of hepatitis C 2018[J]. J Hepatol, 2018, 69(2): 461-511. DOI: 10.1016/j.jhep.2018.03.026. |
| [8] |
Chinese Society of Hepatology, Chinese Medical Association. Chinese guidelines on the management of liver cirrhosis[J]. J Clin Hepatol, 2019, 35(11): 2408-2425. DOI: 10.3969/j.issn.1001-5256.2019.11.006. |
| [9] |
HUI P, COOK DJ, LIM W, et al. The frequency and clinical significance of thrombocytopenia complicating critical illness: A systematic review[J]. Chest, 2011, 139(2): 271-278. DOI: 10.1378/chest.10-2243. |
| [10] |
CHEN YC, TSENG CW, TSENG KC. Rapid platelet count improvement in chronic hepatitis C patients with thrombocytopenia receiving direct-acting antiviral agents[J]. Medicine (Baltimore), 2020, 99(19): e20156. DOI: 10.1097/MD.0000000000020156. |
| [11] |
DIETRICH CF, BAMBER J, BERZIGOTTI A, et al. EFSUMB guidelines and recommendations on the clinical use of liver ultrasound elastography, update 2017 (long version)[J]. Ultraschall Med, 2017, 38(4): e48. DOI: 10.1055/a-0641-0076. |
| [12] |
Chinese Society of Hepatology, Chinese Medical Association, Chinese Society of Infectious Diseases, Chinese Medical Association. Guidelines for the prevention and treatment of hepatitis C(2019 version)[J]. J Clin Hepatol, 2019, 35(12): 2670-2686. DOI: 10.3969/j.issn.1001-5256.2019.12.008. |
| [13] |
EL-SERAG HB, CHRISTIE IC, PUENPATOM A, et al. The effects of sustained virological response to direct-acting anti-viral therapy on the risk of extrahepatic manifestations of hepatitis C infection[J]. Aliment Pharmacol Ther, 2019, 49(11): 1442-1447. DOI: 10.1111/apt.15240. |
| [14] |
HERMOS JA, QUACH L, GAGNON DR, et al. Incident severe thrombocytopenia in veterans treated with pegylated interferon plus ribavirin for chronic hepatitis C infection[J]. Pharmacoepidemiol Drug Saf, 2014, 23(5): 480-488. DOI: 10.1002/pds.3585. |
| [15] |
HUANG CF, YEH ML, HUANG CI, et al. Interference of hepatitis B virus dual infection in platelet count recovery in chronic hepatitis C patients with curative antiviral therapy[J]. J Gastroenterol Hepatol, 2018, 33(5): 1108-1114. DOI: 10.1111/jgh.14017. |
| [16] |
LOUIE KS, MICALLEF JM, PIMENTA JM, et al. Prevalence of thrombocytopenia among patients with chronic hepatitis C: A systematic review[J]. J Viral Hepat, 2011, 18(1): 1-7. DOI: 10.1111/j.1365-2893.2010.01366.x. |
| [17] |
RAWI S, WU GY. Pathogenesis of thrombocytopenia in chronic HCV infection: A review[J]. J Clin Transl Hepatol, 2020, 8(2): 184-191. DOI: 10.14218/JCTH.2020.00007. |
| [18] |
OSADA M, KANEKO M, SAKAMOTO M, et al. Causes of thrombocytopenia in chronic hepatitis C viral infection[J]. Clin Appl Thromb Hemost, 2012, 18(3): 272-280. DOI: 10.1177/1076029611429124. |
| [19] |
AREF S, SLEEM T, EL MENSHAWY N, et al. Antiplatelet antibodies contribute to thrombocytopenia associated with chronic hepatitis C virus infection[J]. Hematology, 2009, 14(5): 277-281. DOI: 10.1179/102453309X439818. |
| [20] |
OGAWA E, FURUSYO N, NAKAMUTA M, et al. Glecaprevir and pibrentasvir for Japanese patients with chronic hepatitis C genotype 1 or 2 infection: Results from a multicenter, real-world cohort study[J]. Hepatol Res, 2019, 49(6): 617-626. DOI: 10.1111/hepr.13328. |
| [21] |
SEKO Y, MORIGUCHI M, TAKAHASHI A, et al. The association between the platelet count and liver volume in compensated cirrhosis patients after the eradication of hepatitis C virus by direct-acting antivirals[J]. Intern Med, 2020, 59(15): 1811-1817. DOI: 10.2169/internalmedicine.4442-20. |
| [22] |
DAI CY, HO CK, HUANG JF, et al. Hepatitis C virus viremia and low platelet count: A study in a hepatitis B & C endemic area in Taiwan[J]. J Hepatol, 2010, 52(2): 160-166. DOI: 10.1016/j.jhep.2009.11.017. |
| [23] |
KAJIHARA M, KATO S, OKAZAKI Y, et al. A role of autoantibody-mediated platelet destruction in thrombocytopenia in patients with cirrhosis[J]. Hepatology, 2003, 37(6): 1267-1276. DOI: 10.1053/jhep.2003.50209. |
| [24] |
HONMA Y, SHIBATA M, HAYASHI T, et al. Effect of direct-acting antivirals on platelet-associated immunoglobulin G and thrombocytopenia in hepatitis C virus-related chronic liver disease[J]. Liver Int, 2019, 39(9): 1641-1651. DOI: 10.1053/jhep.2003.50209. |
| [25] |
SHAO LN, ZHANG ST, WANG N, et al. Platelet indices significantly correlate with liver fibrosis in HCV-infected patients[J]. PLoS One, 2020, 15(1): e0227544. DOI: 10.1371/journal.pone.0227544. |
| [26] |
GOTLIEB N, SCHWARTZ N, ZELBER-SAGI S, et al. Longitudinal decrease in platelet counts as a surrogate marker of liver fibrosis[J]. World J Gastroenterol, 2020, 26(38): 5849-5862. DOI: 10.3748/wjg.v26.i38.5849. |
| [27] |
KODA M, MATUNAGA Y, KAWAKAMI M, et al. FibroIndex, a practical index for predicting significant fibrosis in patients with chronic hepatitis C[J]. Hepatology, 2007, 45(2): 297-306. DOI: 10.1002/hep.21520. |
| [28] |
COVERDALE SA, SAMARASINGHE DA, LIN R, et al. Changes in antipyrine clearance and platelet count, but not conventional liver tests, correlate with fibrotic change in chronic hepatitis C: Value for predicting fibrotic progression[J]. Am J Gastroenterol, 2003, 98(6): 1384-1390. DOI: 10.1111/j.1572-0241.2003.07468.x. |
| [29] |
TANIGUCHI H, IWASAKI Y, FUJIWARA A, et al. Long-term monitoring of platelet count, as a non-invasive marker of hepatic fibrosis progression and/or regression in patients with chronic hepatitis C after interferon therapy[J]. J Gastroenterol Hepatol, 2006, 21(1 Pt 2): 281-287. DOI: 10.1111/j.1440-1746.2006.04201.x. |
| [30] |
CHANG Y, KIM JI, LEE B, et al. Clinical application of ultrasonography-guided percutaneous liver biopsy and its safety over 18 years[J]. Clin Mol Hepatol, 2020, 26(3): 318-327. DOI: 10.3350/cmh.2019.0019n. |
| [31] |
NAKAGOMI R, TATEISHI R, MASUZAKI R, et al. Liver stiffness measurements in chronic hepatitis C: Treatment evaluation and risk assessment[J]. J Gastroenterol Hepatol, 2019, 34(5): 921-928. DOI: 10.1111/jgh.14530. |
| [32] |
WANG JH, CHANGCHIEN CS, HUNG CH, et al. Liver stiffness decrease after effective antiviral therapy in patients with chronic hepatitis C: Longitudinal study using FibroScan[J]. J Gastroenterol Hepatol, 2010, 25(5): 964-969. DOI: 10.1111/j.1440-1746.2009.06194.x. |
| [33] |
ABDEL ALEM S, ELSHARKAWY A, EL AKEL W, et al. Liver stiffness measurements and FIB-4 are predictors of response to sofosbuvir-based treatment regimens in 7256 chronic HCV patients[J]. Expert Rev Gastroenterol Hepatol, 2019, 13(10): 1009-1016. DOI: 10.1080/17474124.2019.1653183. |










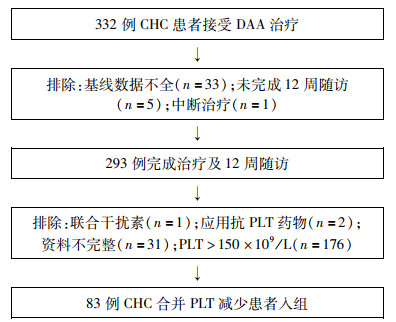




 DownLoad:
DownLoad:
