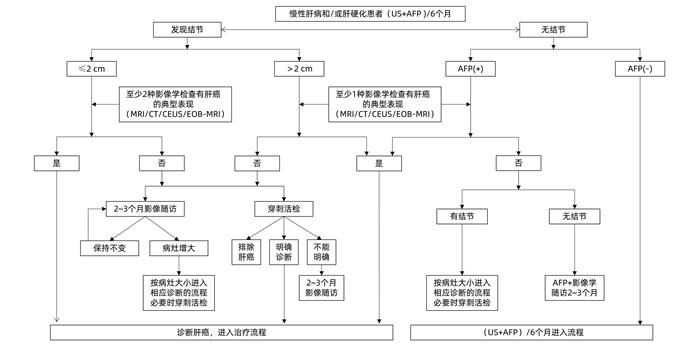
| [1] |
ZHOU M, WANG H, ZENG X, et al. Mortality, morbidity, and risk factors in China and its provinces, 1990-2017: A systematic analysis for the Global Burden of Disease Study 2017[J]. Lancet, 2019, 394(10204): 1145-1158. DOI: 10.1016/S0140-6736(19)30427-1. |
| [2] |
CHEN W, ZHENG R, BAADE PD, et al. Cancer statistics in China, 2015[J]. CA Cancer J Clin, 2016, 66(2): 115-132. DOI: 10.3322/caac.21338. |
| [3] |
|
| [4] |
BRAY F, FERLAY J, SOERJOMATARAM I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA Cancer J Clin, 2018, 68(6): 394-424. DOI: 10.3322/caac.21492. |
| [5] |
GUYATT G, OXMAN AD, AKL EA, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables[J]. J Clin Epidemiol, 2011, 64(4): 383-394. DOI: 10.1016/j.jclinepi.2010.04.026. |
| [6] |
BALSHEM H, HELFAND M, SCHVNEMANN HJ, et al. GRADE guidelines: 3. Rating the quality of evidence[J]. J Clin Epidemiol, 2011, 64(4): 401-406. DOI: 10.1016/j.jclinepi.2010.07.015. |
| [7] |
ANDREWS JC, SCHVNEMANN HJ, OXMAN AD, et al. GRADE guidelines: 15. Going from evidence to recommendation-determinants of a recommendation's direction and strength[J]. J Clin Epidemiol, 2013, 66(7): 726-735. DOI: 10.1016/j.jclinepi.2013.02.003. |
| [8] |
|
| [9] |
ZHANG BH, YANG BH, TANG ZY. Randomized controlled trial of screening for hepatocellular carcinoma[J]. J Cancer Res Clin Oncol, 2004, 130(7): 417-422. DOI: 10.1007/s00432-004-0552-0. |
| [10] |
HOU JL, ZHAO W, LEE C, et al. Outcomes of long-term treatment of chronic HBV infection with entecavir or other agents from a randomized trial in 24 countries[J]. Clin Gastroenterol Hepatol, 2020, 18(2): 457-467. e21. DOI: 10.1016/j.cgh.2019.07.010. |
| [11] |
FAN R, PAPATHEODORIDIS G, SUN J, et al. aMAP risk score predicts hepatocellular carcinoma development in patients with chronic hepatitis[J]. J Hepatol, 2020, 73(6): 1368-1378. DOI: 10.1016/j.jhep.2020.07.025. |
| [12] |
HAO X, FAN R, GUO YB, et al. Establishing an integrated hospital-community pyramid for screening and achieving hepatocellular carcinoma early diagnosis and treatment[J]. Chin J Hepatol, 2021, 29(4): 289-292. DOI: 10.3760/cma.j.cn501113-20210408-00174-1. |
| [13] |
Application of volume navigation guided real time contrast-enhanced ultrasound for diagnosis of small malignant hepatic lesions[J/CD]. Chin J Med Ultrasound(Electronic edition), 2016, 13(1): 56-60. DOI: 10.3877/cma.j.issn.1672-6448.2016.01.014. |
| [14] |
DONG Y, WANG WP, GAN YH, et al. Radiofrequency ablation guided by contrast-enhanced ultrasound for hepatic malignancies: Preliminary results[J]. Clin Radiol, 2014, 69(11): 1129-1135. DOI: 10.1016/j.crad.2014.06.016. |
| [15] |
DONG Y, WANG WP, MAO F, et al. Contrast-enhanced ultrasound features of hepatocellular carcinoma not detected during the screening procedure[J]. Z Gastroenterol, 2017, 55(8): 748-753. DOI: 10.1055/s-0043-114672. |
| [16] |
WANG WP, DONG Y, CAO J, et al. Detection and characterization of small superficially located focal liver lesions by contrast-enhanced ultrasound with high frequency transducers[J]. Med Ultrason, 2017, 19(4): 349-356. DOI: 10.11152/mu-1276. |
| [17] |
DONG Y, WANG WP, MAO F, et al. Application of imaging fusion combining contrast-enhanced ultrasound and magnetic resonance imaging in detection of hepatic cellular carcinomas undetectable by conventional ultrasound[J]. J Gastroenterol Hepatol, 2016, 31(4): 822-828. DOI: 10.1111/jgh.13202. |
| [18] |
DONG Y, WANG WP, XU Y, et al. Point shear wave speed measurement in differentiating benign and malignant focal liver lesions[J]. Med Ultrason, 2017, 19(3): 259-264. DOI: 10.11152/mu-1142. |
| [19] |
CHEN M, CAO J, HU J, et al. Clinical-radiomic analysis for pretreatment prediction of objective response to first transarterial chemoembolization in hepatocellular carcinoma[J]. Liver Cancer, 2021, 10(1): 38-51. DOI: 10.1159/000512028. |
| [20] |
LEE YJ, LEE JM, LEE JS, et al. Hepatocellular carcinoma: Diagnostic performance of multidetector CT and MR imaging-a systematic review and meta-analysis[J]. Radiology, 2015, 275(1): 97-109. DOI: 10.1148/radiol.14140690. |
| [21] |
LIU X, JIANG H, CHEN J, et al. Gadoxetic acid disodium-enhanced magnetic resonance imaging outperformed multidetector computed tomography in diagnosing small hepatocellular carcinoma: A meta-analysis[J]. Liver Transpl, 2017, 23(12): 1505-1518. DOI: 10.1002/lt.24867. |
| [22] |
MARRERO JA, KULIK LM, SIRLIN CB, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases[J]. Hepatology, 2018, 68(2): 723-750. DOI: 10.1002/hep.29913. |
| [23] |
VOGEL A, CERVANTES A, CHAU I, et al. Hepatocellular carcinoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up[J]. Ann Oncol, 2018, 29(Suppl 4): iv238-iv255. DOI: 10.1093/annonc/mdy308.29(Suppl4):iv238-iv55. |
| [24] |
OMATA M, CHENG AL, KOKUDO N, et al. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: A 2017 update[J]. Hepatol Int, 2017, 11(4): 317-370. DOI: 10.1007/s12072-017-9799-9. |
| [25] |
CHO ES, CHOI JY. MRI features of hepatocellular carcinoma related to biologic behavior[J]. Korean J Radiol, 2015, 16(3): 449-464. DOI: 10.3348/kjr.2015.16.3.449. |
| [26] |
HWANG J, KIM YK, JEONG WK, et al. Nonhypervascular hypointense nodules at gadoxetic acid-enhanced MR imaging in chronic liver disease: Diffusion-weighted imaging for characterization[J]. Radiology, 2015, 276(1): 137-146. DOI: 10.1148/radiol.15141350. |
| [27] |
ZENG MS, YE HY, GUO L, et al. Gd-EOB-DTPA-enhanced magnetic resonance imaging for focal liver lesions in Chinese patients: A multicenter, open-label, phase Ⅲ study[J]. Hepatobiliary Pancreat Dis Int, 2013, 12(6): 607-616. DOI: 10.1016/s1499-3872(13)60096-x. |
| [28] |
ICHIKAWA T, SAITO K, YOSHIOKA N, et al. Detection and characterization of focal liver lesions: A Japanese phase Ⅲ, multicenter comparison between gadoxetic acid disodium-enhanced magnetic resonance imaging and contrast-enhanced computed tomography predominantly in patients with hepatocellular carcinoma and chronic liver disease[J]. Invest Radiol, 2010, 45(3): 133-141. DOI: 10.1097/RLI.0b013e3181caea5b. |
| [29] |
WANG W, YANG C, ZHU K, et al. Recurrence after curative resection of hepatitis B virus-related hepatocellular carcinoma: Diagnostic algorithms on gadoxetic acid-enhanced magnetic resonance imaging[J]. Liver Transpl, 2020, 26(6): 751-763. DOI: 10.1002/lt.25713. |
| [30] |
YOO SH, CHOI JY, JANG JW, et al. Gd-EOB-DTPA-enhanced MRI is better than MDCT in decision making of curative treatment for hepatocellular carcinoma[J]. Ann Surg Oncol, 2013, 20(9): 2893-2900. DOI: 10.1245/s10434-013-3001-y. |
| [31] |
RAO SX, WANG J, WANG J, et al. Chinese consensus on the clinical application of hepatobiliary magnetic resonance imaging contrast agent: Gadoxetic acid disodium[J]. J Dig Dis, 2019, 20(2): 54-61. DOI: 10.1111/1751-2980.12707. |
| [32] |
RENZULLI M, BISELLI M, BROCCHI S, et al. New hallmark of hepatocellular carcinoma, early hepatocellular carcinoma and high-grade dysplastic nodules on Gd-EOB-DTPA MRI in patients with cirrhosis: A new diagnostic algorithm[J]. Gut, 2018, 67(9): 1674-1682. DOI: 10.1136/gutjnl-2017-315384. |
| [33] |
XU X, ZHANG HL, LIU QP, et al. Radiomic analysis of contrast-enhanced CT predicts microvascular invasion and outcome in hepatocellular carcinoma[J]. J Hepatol, 2019, 70(6): 1133-1144. DOI: 10.1016/j.jhep.2019.02.023. |
| [34] |
CHONG HH, YANG L, SHENG RF, et al. Multi-scale and multi-parametric radiomics of gadoxetate disodium-enhanced MRI predicts microvascular invasion and outcome in patients with solitary hepatocellular carcinoma ≤ 5 cm[J]. Eur Radiol, 2021, 31(7): 4824-4838. DOI: 10.1007/s00330-020-07601-2. |
| [35] |
YANG L, GU D, WEI J, et al. A radiomics nomogram for preoperative prediction of microvascular invasion in hepatocellular carcinoma[J]. Liver Cancer, 2019, 8(5): 373-386. DOI: 10.1159/000494099. |
| [36] |
LEI Z, LI J, WU D, et al. Nomogram for preoperative estimation of microvascular invasion risk in hepatitis B virus-related hepatocellular carcinoma within the milan criteria[J]. JAMA Surg, 2016, 151(4): 356-363. DOI: 10.1001/jamasurg.2015.4257. |
| [37] |
LIN CY, CHEN JH, LIANG JA, et al. 18F-FDG PET or PET/CT for detecting extrahepatic metastases or recurrent hepatocellular carcinoma: A systematic review and meta-analysis[J]. Eur J Radiol, 2012, 81(9): 2417-2422. DOI: 10.1016/j.ejrad.2011.08.004. |
| [38] |
PARK JW, KIM JH, KIM SK, et al. A prospective evaluation of 18F-FDG and 11C-acetate PET/CT for detection of primary and metastatic hepatocellular carcinoma[J]. J Nucl Med, 2008, 49(12): 1912-1921. DOI: 10.2967/jnumed.108.055087. |
| [39] |
BOELLAARD R, O'Doherty MJ, DOHERTY MJ, et al. FDG PET and PET/CT: EANM procedure guidelines for tumour PET imaging: Version 1.0[J]. Eur J Nucl Med Mol Imaging, 2010, 37(1): 181-200. DOI: 10.1007/s00259-009-1297-4. |
| [40] |
CHALIAN H, TÖRE HG, HOROWITZ JM, et al. Radiologic assessment of response to therapy: Comparison of RECIST Versions 1.1 and 1.0[J]. Radiographics, 2011, 31(7): 2093-2105. DOI: 10.1148/rg.317115050. |
| [41] |
WAHL RL, JACENE H, KASAMON Y, et al. From RECIST to PERCIST: Evolving considerations for PET response criteria in solid tumors[J]. J Nucl Med, 2009, 50 Suppl 1(Suppl 1): 122S-50S. DOI: 10.2967/jnumed.108.057307. |
| [42] |
FERDA J, FERDOVÁ E, BAXA J, et al. The role of 18F-FDG accumulation and arterial enhancement as biomarkers in the assessment of typing, grading and staging of hepatocellular carcinoma using 18F-FDG-PET/CT with integrated dual-phase CT angiography[J]. Anticancer Res, 2015, 35(4): 2241-2246.
|
| [43] |
HYUN SH, EO JS, LEE JW, et al. Prognostic value of 18F-fluorodeoxyglucose positron emission tomography/computed tomography in patients with Barcelona Clinic Liver Cancer stages 0 and A hepatocellular carcinomas: A multicenter retrospective cohort study[J]. Eur J Nucl Med Mol Imaging, 2016, 43(9): 1638-1645. DOI: 10.1007/s00259-016-3348-y. |
| [44] |
LEE JW, OH JK, CHUNG YA, et al. Prognostic significance of 18F-FDG uptake in hepatocellular carcinoma treated with transarterial chemoembolization or concurrent chemoradiotherapy: A multicenter retrospective cohort study[J]. J Nucl Med, 2016, 57(4): 509-516. DOI: 10.2967/jnumed.115.167338. |
| [45] |
NA SJ, OH JK, HYUN SH, et al. (18)F-FDG PET/CT can predict survival of advanced hepatocellular carcinoma patients: A multicenter retrospective cohort study[J]. J Nucl Med, 2017, 58(5): 730-736. DOI: 10.2967/jnumed.116.182022. |
| [46] |
BERTAGNA F, BERTOLI M, BOSIO G, et al. Diagnostic role of radiolabelled choline PET or PET/CT in hepatocellular carcinoma: A systematic review and meta-analysis[J]. Hepatol Int, 2014, 8(4): 493-500. DOI: 10.1007/s12072-014-9566-0. |
| [47] |
CHEUNG TT, HO CL, LO CM, et al. 11C-acetate and 18F-FDG PET/CT for clinical staging and selection of patients with hepatocellular carcinoma for liver transplantation on the basis of Milan criteria: Surgeon's perspective[J]. J Nucl Med, 2013, 54(2): 192-200. DOI: 10.2967/jnumed.112.107516. |
| [48] |
ZHANG Y, SHI H, CHENG D, et al. Added value of SPECT/spiral CT versus SPECT in diagnosing solitary spinal lesions in patients with extraskeletal malignancies[J]. Nucl Med Commun, 2013, 34(5): 451-458. DOI: 10.1097/MNM.0b013e32835fa552. |
| [49] |
HECTORS SJ, WAGNER M, BESA C, et al. Multiparametric FDG-PET/MRI of hepatocellular carcinoma: Initial experience[J]. Contrast Media Mol Imaging, 2018, 2018: 5638283. DOI: 10.1155/2018/5638283. |
| [50] |
ZHOU J, YU L, GAO X, et al. Plasma microRNA panel to diagnose hepatitis B virus-related hepatocellular carcinoma[J]. J Clin Oncol, 2011, 29(36): 4781-4788. DOI: 10.1200/JCO.2011.38.2697. |
| [51] |
BEST J, BECHMANN LP, SOWA JP, et al. GALAD score detects early hepatocellular carcinoma in an international cohort of patients with nonalcoholic steatohepatitis[J]. Clin Gastroenterol Hepatol, 2020, 18(3): 728-735. e4. DOI: 10.1016/j.cgh.2019.11.012. |
| [52] |
FORNER A, VILANA R, AYUSO C, et al. Diagnosis of hepatic nodules 20 mm or smaller in cirrhosis: Prospective validation of the noninvasive diagnostic criteria for hepatocellular carcinoma[J]. Hepatology, 2008, 47(1): 97-104. DOI: 10.1002/hep.21966. |
| [53] |
ROBERTS LR, SIRLIN CB, ZAIEM F, et al. Imaging for the diagnosis of hepatocellular carcinoma: A systematic review and meta-analysis[J]. Hepatology, 2018, 67(1): 401-421. DOI: 10.1002/hep.29487. |
| [54] |
European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma[J]. J Hepatol, 2018, 69(1): 182-236. DOI: 10.1016/j.jhep.2018.03.019. |
| [55] |
HUANG YH, ZHANG CZ, HUANG QS, et al. Clinicopathologic features, tumor immune microenvironment and genomic landscape of Epstein-Barr virus-associated intrahepatic cholangiocarcinoma[J]. J Hepatol, 2021, 74(4): 838-849. DOI: 10.1016/j.jhep.2020.10.037. |
| [56] |
YU WL, YU G, DONG H, et al. Proteomics analysis identified TPI1 as a novel biomarker for predicting recurrence of intrahepatic cholangiocarcinoma[J]. J Gastroenterol, 2020, 55(12): 1171-1182. DOI: 10.1007/s00535-020-01729-0. |
| [57] |
PARADIS V, FUKAYAMA M, PARK Y, et al. Tumours of liver and intrahepatic bile ducts[M]. 5th ed. Lyon, France: IARC Press, 2019.
|
| [58] |
CONG WM. Surgical pathology of hepatobiliary tumors[M]. 1st ed. Singapore: Springer, 2017.
|
| [59] |
CONG WM, BU H, CHEN J, et al. Practice guidelines for the pathological diagnosis of primary liver cancer: 2015 update[J]. World J Gastroenterol, 2016, 22(42): 9279-9287. DOI: 10.3748/wjg.v22.i42.9279. |
| [60] |
CHEN L, CHEN S, ZHOU Q, et al. Microvascular invasion status and its survival impact in hepatocellular carcinoma depend on tissue sampling protocol[J]. Ann Surg Oncol, 2021, 28(11): 6747-6757. DOI: 10.1245/s10434-021-09673-w. |
| [61] |
NARA S, SHIMADA K, SAKAMOTO Y, et al. Prognostic impact of marginal resection for patients with solitary hepatocellular carcinoma: Evidence from 570 hepatectomies[J]. Surgery, 2012, 151(4): 526-536. DOI: 10.1016/j.surg.2011.12.002. |
| [62] |
CONG WM. Surgical pathology of hepatobiliary tumors[M]. Beijing: People's Medical Publishing House, 2015: 276-320.
丛文铭. 肝胆肿瘤外科病理学[M]. 北京: 人民卫生出版社, 2015: 276-320.
|
| [63] |
SCHEUER PJ. Classification of chronic viral hepatitis: a need for reassessment[J]. J Hepatol, 1991, 13(3): 372-374. DOI: 10.1016/0168-8278(91)90084-o. |
| [64] |
Chinese Society of Infectious Diseases and Parasitology and Chinese Society of Hepatology of Chinese Medical Association. Regimens for prevention and treatment of viral hepatitis[J]. Chin J Infect Dis, 2001, 19(1): 56-62. DOI: 10.3760/cma.j.issn.1671-7600.2001.01.025. |
| [65] |
World Health Organization. Guidelines for the prevention, care and treatment of persons with chronic hepatitis B infection[R]. Geneva: World Health Organization, 2015.
|
| [66] |
RODRÍGUEZ-PERÁLVAREZ M, LUONG TV, ANDREANA L, et al. A systematic review of microvascular invasion in hepatocellular carcinoma: Diagnostic and prognostic variability[J]. Ann Surg Oncol, 2013, 20(1): 325-339. DOI: 10.1245/s10434-012-2513-1. |
| [67] |
Chinese Society of Liver Cancer, Chinese Anti-Cancer Association; Liver Cancer Study Group, Chinese Society of Hepatology, Chinese Medical Association; Chinese Society of Pathology, Chinese Anti-Cancer Association; et al. Evidence-based practice guidelines for the standardized pathological diagnosis of primary liver cancer in China(2015 update)[J]. J Clin Hepatol, 2015, 31(6): 833-839. DOI: 10.3969/j.issn.1001-5256.2015.06.004. |
| [68] |
SHENG X, JI Y, REN GP, et al. A standardized pathological proposal for evaluating microvascular invasion of hepatocellular carcinoma: A multicenter study by LCPGC[J]. Hepatol Int, 2020, 14(6): 1034-1047. DOI: 10.1007/s12072-020-10111-4. |
| [69] |
ISIK B, GONULTAS F, SAHIN T, et al. Microvascular venous invasion in hepatocellular carcinoma: Why do recurrences occur?[J]. J Gastrointest Cancer, 2020, 51(4): 1133-1136. DOI: 10.1007/s12029-020-00487-9. |
| [70] |
ZHANG X, LI J, SHEN F, et al. Significance of presence of microvascular invasion in specimens obtained after surgical treatment of hepatocellular carcinoma[J]. J Gastroenterol Hepatol, 2018, 33(2): 347-354. DOI: 10.1111/jgh.13843. |
| [71] |
TRAVIS WD, DACIC S, WISTUBA I, et al. IASLC multidisciplinary recommendations for pathologic assessment of lung cancer resection specimens after neoadjuvant therapy[J]. J Thorac Oncol, 2020, 15(5): 709-740. DOI: 10.1016/j.jtho.2020.01.005. |
| [72] |
ALLARD MA, SEBAGH M, RUIZ A, et al. Does pathological response after transarterial chemoembolization for hepatocellular carcinoma in cirrhotic patients with cirrhosis predict outcome after liver resection or transplantation?[J]. J Hepatol, 2015, 63(1): 83-92. DOI: 10.1016/j.jhep.2015.01.023. |
| [73] |
STEIN JE, LIPSON EJ, COTTRELL TR, et al. Pan-tumor pathologic scoring of response to PD-(L)1 blockade[J]. Clin Cancer Res, 2020, 26(3): 545-551. DOI: 10.1158/1078-0432.CCR-19-2379. |
| [74] |
IMAMURA H, SEYAMA Y, KOKUDO N, et al. One thousand fifty-six hepatectomies without mortality in 8 years[J]. Arch Surg, 2003, 138(11): 1198-1206; discussion 1206. DOI: 10.1001/archsurg.138.11.1198. |
| [75] |
KUBOTA K, MAKUUCHI M, KUSAKA K, et al. Measurement of liver volume and hepatic functional reserve as a guide to decision-making in resectional surgery for hepatic tumors[J]. Hepatology, 1997, 26(5): 1176-1181. DOI: 10.1053/jhep.1997.v26.pm0009362359. |
| [76] |
BRUIX J, CASTELLS A, BOSCH J, et al. Surgical resection of hepatocellular carcinoma in cirrhotic patients: Prognostic value of preoperative portal pressure[J]. Gastroenterology, 1996, 111(4): 1018-1022. DOI: 10.1016/s0016-5085(96)70070-7. |
| [77] |
CESCON M, COLECCHIA A, CUCCHETTI A, et al. Value of transient elastography measured with FibroScan in predicting the outcome of hepatic resection for hepatocellular carcinoma[J]. Ann Surg, 2012, 256(5): 706-712; discussion 712-713. DOI: 10.1097/SLA.0b013e3182724ce8. |
| [78] |
SHEN Y, ZHOU C, ZHU G, et al. Liver stiffness assessed by shear wave elastography predicts postoperative liver failure in patients with hepatocellular carcinoma[J]. J Gastrointest Surg, 2017, 21(9): 1471-1479. DOI: 10.1007/s11605-017-3443-9. |
| [79] |
RAJAKANNU M, CHERQUI D, CIACIO O, et al. Liver stiffness measurement by transient elastography predicts late posthepatectomy outcomes in patients undergoing resection for hepatocellular carcinoma[J]. Surgery, 2017, 162(4): 766-774. DOI: 10.1016/j.surg.2017.06.006. |
| [80] |
ZHONG JH, KE Y, GONG WF, et al. Hepatic resection associated with good survival for selected patients with intermediate and advanced-stage hepatocellular carcinoma[J]. Ann Surg, 2014, 260(2): 329-340. DOI: 10.1097/SLA.0000000000000236. |
| [81] |
XIAO H, ZHANG B, MEI B, et al. Hepatic resection for hepatocellular carcinoma in patients with portal hypertension: A long-term benefit compared with transarterial chemoembolization and thermal ablation[J]. Medicine (Baltimore), 2015, 94(7): e495. DOI: 10.1097/MD.0000000000000495. |
| [82] |
BOSCH J, ABRALDES JG, BERZIGOTTI A, et al. The clinical use of HVPG measurements in chronic liver disease[J]. Nat Rev Gastroenterol Hepatol, 2009, 6(10): 573-582. DOI: 10.1038/nrgastro.2009.149. |
| [83] |
CHEN X, ZHAI J, CAI X, et al. Severity of portal hypertension and prediction of postoperative liver failure after liver resection in patients with Child-Pugh grade A cirrhosis[J]. Br J Surg, 2012, 99(12): 1701-1710. DOI: 10.1002/bjs.8951. |
| [84] |
CHEN MS, LI JQ, ZHENG Y, et al. A prospective randomized trial comparing percutaneous local ablative therapy and partial hepatectomy for small hepatocellular carcinoma[J]. Ann Surg, 2006, 243(3): 321-328. DOI: 10.1097/01.sla.0000201480.65519.b8. |
| [85] |
KUDO M, HASEGAWA K, KAWAGUCHI Y, et al. A multicenter randomized controlled trial to evaluate the efficacy of surgery versus radiofrequency ablation for small hepatocellular carcinoma (SURF trial): Analysis of overall survival[J]. J Clin Oncol, 2021, 39(15_suppl): 4093. DOI: 10.1200/JCO.2021.39.15_suppl.4093 |
| [86] |
MOHKAM K, DUMONT PN, MANICHON AF, et al. No-touch multibipolar radiofrequency ablation vs. surgical resection for solitary hepatocellular carcinoma ranging from 2 to 5 cm[J]. J Hepatol, 2018, 68(6): 1172-1180. DOI: 10.1016/j.jhep.2018.01.014. |
| [87] |
XU XL, LIU XD, LIANG M, et al. Radiofrequency ablation versus hepatic resection for small hepatocellular carcinoma: Systematic review of randomized controlled trials with meta-analysis and trial sequential analysis[J]. Radiology, 2018, 287(2): 461-472. DOI: 10.1148/radiol.2017162756. |
| [88] |
LIU PH, HSU CY, HSIA CY, et al. Surgical resection versus radiofrequency ablation for single hepatocellular carcinoma ≤2 cm in a propensity score model[J]. Ann Surg, 2016, 263(3): 538-545. DOI: 10.1097/SLA.0000000000001178. |
| [89] |
FENG K, YAN J, LI X, et al. A randomized controlled trial of radiofrequency ablation and surgical resection in the treatment of small hepatocellular carcinoma[J]. J Hepatol, 2012, 57(4): 794-802. DOI: 10.1016/j.jhep.2012.05.007. |
| [90] |
XU Q, KOBAYASHI S, YE X, et al. Comparison of hepatic resection and radiofrequency ablation for small hepatocellular carcinoma: A meta-analysis of 16, 103 patients[J]. Sci Rep, 2014, 4: 7252. DOI: 10.1038/srep07252. |
| [91] |
XIA Y, LI J, LIU G, et al. Long-term effects of repeat hepatectomy vs percutaneous radiofrequency ablation among patients with recurrent hepatocellular carcinoma: A randomized clinical trial[J]. JAMA Oncol, 2020, 6(2): 255-263. DOI: 10.1001/jamaoncol.2019.4477. |
| [92] |
YIN L, LI H, LI AJ, et al. Partial hepatectomy vs. transcatheter arterial chemoembolization for resectable multiple hepatocellular carcinoma beyond Milan Criteria: A RCT[J]. J Hepatol, 2014, 61(1): 82-88. DOI: 10.1016/j.jhep.2014.03.012. |
| [93] |
TORZILLI G, BELGHITI J, KOKUDO N, et al. A snapshot of the effective indications and results of surgery for hepatocellular carcinoma in tertiary referral centers: Is it adherent to the EASL/AASLD recommendations?: An observational study of the HCC East-West study group[J]. Ann Surg, 2013, 257(5): 929-937. DOI: 10.1097/SLA.0b013e31828329b8. |
| [94] |
WANG K, GUO WX, CHEN MS, et al. Multimodality treatment for hepatocellular carcinoma with portal vein tumor thrombus: A large-scale, multicenter, propensity mathching score analysis[J]. Medicine (Baltimore), 2016, 95(11): e3015. DOI: 10.1097/MD.0000000000003015. |
| [95] |
WEI X, JIANG Y, ZHANG X, et al. Neoadjuvant three-dimensional conformal radiotherapy for resectable hepatocellular carcinoma with portal vein tumor thrombus: A randomized, open-label, multicenter controlled study[J]. J Clin Oncol, 2019, 37(24): 2141-2151. DOI: 10.1200/JCO.18.02184. |
| [96] |
LI XL, ZHU XD, CAI H, et al. Postoperative α-fetoprotein response predicts tumor recurrence and survival after hepatectomy for hepatocellular carcinoma: A propensity score matching analysis[J]. Surgery, 2019, 165(6): 1161-1167. DOI: 10.1016/j.surg.2019.01.009. |
| [97] |
YANG J, TAO HS, CAI W, et al. Accuracy of actual resected liver volume in anatomical liver resections guided by 3-dimensional parenchymal staining using fusion indocyanine green fluorescence imaging[J]. J Surg Oncol, 2018, 118(7): 1081-1087. DOI: 10.1002/jso.25258. |
| [98] |
MISE Y, HASEGAWA K, SATOU S, et al. How has virtual hepatectomy changed the practice of liver surgery?: Experience of 1194 virtual hepatectomy before liver resection and living donor liver transplantation[J]. Ann Surg, 2018, 268(1): 127-133. DOI: 10.1097/SLA.0000000000002213. |
| [99] |
Chinese Society of Digital Medicine, Chinese Medical Association; Digital Intelligent Surgery Professional Committee, Chinese Research Hospital Association; Chinese Society of Liver Cancer, Chinese Medical Doctor Association, et al. Guidelines for the application of computer-assisted indocyanine green molecular fluorescence imaging technology in liver tumor diagnosis and surgical navigation (2019 edition)[J]. Chin J Pract Surg, 2019, 39(7): 641-650, 654. DOI: 10.19538/j.cjps.issn1005-2208.2019.07.01. |
| [100] |
JIANG HT, CAO JY. Impact of laparoscopic versus open hepatectomy on perioperative clinical outcomes of patients with primary hepatic carcinoma[J]. Chin Med Sci J, 2015, 30(2): 80-83. DOI: 10.1016/s1001-9294(15)30016-x. |
| [101] |
Pancreatic Disease Committee of Chinese Research Hospital Association. Chinese expert consensus on laparoscopic hepatectomy for hepatocellular carcinoma (2020 edtion)[J]. Chin J Dig Surg, 2020, 19(11): 1119-1134. DOI: 10.3760/cma.j.cn115610-20201029-00682. |
| [102] |
WANG X, TEH C, ISHIZAWA T, et al. Consensus guidelines for the use of fluorescence imaging in hepatobiliary surgery[J]. Ann Surg, 2021, 274(1): 97-106. DOI: 10.1097/SLA.0000000000004718. |
| [103] |
|
| [104] |
HIDAKA M, EGUCHI S, OKUDA K, et al. Impact of anatomical resection for hepatocellular carcinoma with microportal invasion (vp1): A multi-institutional study by the kyushu study group of liver surgery[J]. Ann Surg, 2020, 271(2): 339-346. DOI: 10.1097/SLA.0000000000002981. |
| [105] |
ZHONG FP, ZHANG YJ, LIU Y, et al. Prognostic impact of surgical margin in patients with hepatocellular carcinoma: A meta-analysis[J]. Medicine (Baltimore), 2017, 96(37): e8043. DOI: 10.1097/MD.0000000000008043. |
| [106] |
SHI M, GUO RP, LIN XJ, et al. Partial hepatectomy with wide versus narrow resection margin for solitary hepatocellular carcinoma: A prospective randomized trial[J]. Ann Surg, 2007, 245(1): 36-43. DOI: 10.1097/01.sla.0000231758.07868.71. |
| [107] |
YANG P, SI A, YANG J, et al. A wide-margin liver resection improves long-term outcomes for patients with HBV-related hepatocellular carcinoma with microvascular invasion[J]. Surgery, 2019, 165(4): 721-730. DOI: 10.1016/j.surg.2018.09.016. |
| [108] |
LIU CL, FAN ST, LO CM, et al. Anterior approach for major right hepatic resection for large hepatocellular carcinoma[J]. Ann Surg, 2000, 232(1): 25-31. DOI: 10.1097/00000658-200007000-00004. |
| [109] |
ZHOU C, PENG Y, ZHOU K, et al. Surgical resection plus radiofrequency ablation for the treatment of multifocal hepatocellular carcinoma[J]. Hepatobiliary Surg Nutr, 2019, 8(1): 19-28. DOI: 10.21037/hbsn.2018.11.19. |
| [110] |
ZHANG ZM, LAI EC, ZHANG C, et al. The strategies for treating primary hepatocellular carcinoma with portal vein tumor thrombus[J]. Int J Surg, 2015, 20: 8-16. DOI: 10.1016/j.ijsu.2015.05.009. |
| [111] |
FU SY, LAU WY, LI AJ, et al. Liver resection under total vascular exclusion with or without preceding Pringle manoeuvre[J]. Br J Surg, 2010, 97(1): 50-55. DOI: 10.1002/bjs.6841. |
| [112] |
SATOH S, IKAI I, HONDA G, et al. Clinicopathologic evaluation of hepatocellular carcinoma with bile duct thrombi[J]. Surgery, 2000, 128(5): 779-783. DOI: 10.1067/msy.2000.108659. |
| [113] |
KIM DS, KIM BW, HATANO E, et al. Surgical outcomes of hepatocellular carcinoma with bile duct tumor thrombus: A Korea-Japan multicenter study[J]. Ann Surg, 2020, 271(5): 913-921. DOI: 10.1097/SLA.0000000000003014. |
| [114] |
ZHU XD, HUANG C, SHEN YH, et al. Downstaging and resection of initially unresectable hepatocellular carcinoma with tyrosine kinase inhibitor and anti-PD-1 antibody combinations[J]. Liver Cancer, 2021, 10(4): 320-329. DOI: 10.1159/000514313. |
| [115] |
|
| [116] |
ZHANG WW, HU BY, HAN J, et al. Preliminary report on the study of conversion therapy of advanced hepatocellular carcinoma combined PD-1 inhibitors with multi-target tyrosine kinase inhibitors[J]. Chin J Hepatobiliary Surg, 2020, 26(12): 947-948. DOI: 10.3760/cma.j.cn113884-20201203-00611. |
| [117] |
HE M, LI Q, ZOU R, et al. Sorafenib plus hepatic arterial infusion of oxaliplatin, fluorouracil, and leucovorin vs sorafenib alone for hepatocellular carcinoma with portal vein invasion: A randomized clinical trial[J]. JAMA Oncol, 2019, 5(7): 953-960. DOI: 10.1001/jamaoncol.2019.0250. |
| [118] |
CHEN X, ZHANG Y, ZHANG N, et al. Lenvatinib combined nivolumab injection followed by extended right hepatectomy is a feasible treatment for patients with massive hepatocellular carcinoma: A case report[J]. Onco Targets Ther, 2019, 12: 7355-7359. DOI: 10.2147/OTT.S217123. |
| [119] |
HE MK, LE Y, LI QJ, et al. Hepatic artery infusion chemotherapy using mFOLFOX versus transarterial chemoembolization for massive unresectable hepatocellular carcinoma: A prospective non-randomized study[J]. Chin J Cancer, 2017, 36(1): 83. DOI: 10.1186/s40880-017-0251-2. |
| [120] |
ZHANG Y, HUANG G, WANG Y, et al. IS salvage liver resection necessary for initially unresectable hepatocellular carcinoma patients downstaged by transarterial chemoembolization? Ten years of experience[J]. Oncologist, 2016, 21(12): 1442-1449. DOI: 10.1634/theoncologist.2016-0094. |
| [121] |
LYU N, KONG Y, MU L, et al. Hepatic arterial infusion of oxaliplatin plus fluorouracil/leucovorin vs. sorafenib for advanced hepatocellular carcinoma[J]. J Hepatol, 2018, 69(1): 60-69. DOI: 10.1016/j.jhep.2018.02.008. |
| [122] |
BYUN HK, KIM HJ, IM YR, et al. Dose escalation by intensity modulated radiotherapy in liver-directed concurrent chemoradiotherapy for locally advanced BCLC stage C hepatocellular carcinoma[J]. Radiother Oncol, 2019, 133: 1-8. DOI: 10.1016/j.radonc.2018.12.025. |
| [123] |
LI B, QIU J, ZHENG Y, et al. Conversion to resectability using transarterial chemoembolization combined with hepatic arterial infusion chemotherapy for initially unresectable hepatocellular carcinoma[J]. Ann Surg Open, 2021, 2(2): e057. DOI: 10.1097/AS9.0000000000000057. |
| [124] |
HE MK, LIANG RB, ZHAO Y, et al. Lenvatinib, toripalimab, plus hepatic arterial infusion chemotherapy versus lenvatinib alone for advanced hepatocellular carcinoma[J]. Ther Adv Med Oncol, 2021, 13: 17588359211002720. DOI: 10.1177/17588359211002720. |
| [125] |
WAKABAYASHI H, OKADA S, MAEBA T, et al. Effect of preoperative portal vein embolization on major hepatectomy for advanced-stage hepatocellular carcinomas in injured livers: A preliminary report[J]. Surg Today, 1997, 27(5): 403-410. DOI: 10.1007/BF02385702. |
| [126] |
ZHENG SG, LI JW, XIAO L, et al. Totally laparoscopic associating liver partition and portal vein ligation for staged hepatectomy for the treatment of cirrhotic hepatocellular carcinoma[J]. Chin J Dig Surg, 2014, 13(7): 502-507. DOI: 10.3760/cma.j.issn.1673-9752.2014.07.002. |
| [127] |
HONG DE F, ZHANG YB, PENG SY, et al. Percutaneous microwave ablation liver partition and portal vein embolization for rapid liver regeneration: A minimally invasive first step of ALPPS for hepatocellular carcinoma[J]. Ann Surg, 2016, 264(1): e1-e2. DOI: 10.1097/SLA.0000000000001707. |
| [128] |
D'HAESE JG, NEUMANN J, WENIGER M, et al. Should ALPPS be used for liver resection in intermediate-stage HCC?[J] Ann Surg Oncol, 2016, 23(4): 1335-1343. DOI: 10.1245/s10434-015-5007-0. |
| [129] |
LI PP, HUANG G, JIA NY, et al. Associating liver partition and portal vein ligation for staged hepatectomy versus sequential transarterial chemoembolization and portal vein embolization in staged hepatectomy for HBV-related hepatocellular carcinoma: A randomized comparative study[J]. Hepatobiliary Surg Nutr, 2022, 11(1): 38-51. DOI: 10.21037/hbsn-20-264. |
| [130] |
WANG Z, PENG Y, HU J, et al. Associating liver partition and portal vein ligation for staged hepatectomy for unresectable hepatitis B virus-related hepatocellular carcinoma: A single center study of 45 patients[J]. Ann Surg, 2020, 271(3): 534-541. DOI: 10.1097/SLA.0000000000002942. |
| [131] |
SHI HY, WANG SN, WANG SC, et al. Preoperative transarterial chemoembolization and resection for hepatocellular carcinoma: A nationwide Taiwan database analysis of long-term outcome predictors[J]. J Surg Oncol, 2014, 109(5): 487-493. DOI: 10.1002/jso.23521. |
| [132] |
ZHOU WP, LAI EC, LI AJ, et al. A prospective, randomized, controlled trial of preoperative transarterial chemoembolization for resectable large hepatocellular carcinoma[J]. Ann Surg, 2009, 249(2): 195-202. DOI: 10.1097/SLA.0b013e3181961c16. |
| [133] |
KASEB AO, CAO HST, MOHAMED YI, et al. Final results of a randomized, open label, perioperative phase Ⅱ study evaluating nivolumab alone or nivolumab plus ipilimumab in patients with resectable HCC[J]. J Clin Oncol, 2020, 38(15_suppl): 4599. DOI: 10.1200/JCO.2020.38.15_suppl.4599 |
| [134] |
WANG Z, REN Z, CHEN Y, et al. Adjuvant transarterial chemoembolization for HBV-related hepatocellular carcinoma after resection: A randomized controlled study[J]. Clin Cancer Res, 2018, 24(9): 2074-2081. DOI: 10.1158/1078-0432.CCR-17-2899. |
| [135] |
WEI W, JIAN PE, LI SH, et al. Adjuvant transcatheter arterial chemoembolization after curative resection for hepatocellular carcinoma patients with solitary tumor and microvascular invasion: A randomized clinical trial of efficacy and safety[J]. Cancer Commun (Lond), 2018, 38(1): 61. DOI: 10.1186/s40880-018-0331-y. |
| [136] |
CHEN Q, SHU C, LAURENCE AD, et al. Effect of Huaier granule on recurrence after curative resection of HCC: A multicentre, randomised clinical trial[J]. Gut, 2018, 67(11): 2006-2016. DOI: 10.1136/gutjnl-2018-315983. |
| [137] |
HUANG G, LI PP, LAU WY, et al. Antiviral therapy reduces hepatocellular carcinoma recurrence in patients with low HBV-DNA levels: A randomized controlled trial[J]. Ann Surg, 2018, 268(6): 943-954. DOI: 10.1097/SLA.0000000000002727. |
| [138] |
YIN J, LI N, HAN Y, et al. Effect of antiviral treatment with nucleotide/nucleoside analogs on postoperative prognosis of hepatitis B virus-related hepatocellular carcinoma: A two-stage longitudinal clinical study[J]. J Clin Oncol, 2013, 31(29): 3647-3655. DOI: 10.1200/JCO.2012.48.5896. |
| [139] |
HUANG G, LAU WY, WANG ZG, et al. Antiviral therapy improves postoperative survival in patients with hepatocellular carcinoma: A randomized controlled trial[J]. Ann Surg, 2015, 261(1): 56-66. DOI: 10.1097/SLA.0000000000000858. |
| [140] |
SINGAL AG, LIM JK, KANWAL F. AGA Clinical practice update on interaction between oral direct-acting antivirals for chronic hepatitis C infection and hepatocellular carcinoma: Expert review[J]. Gastroenterology, 2019, 156(8): 2149-2157. DOI: 10.1053/j.gastro.2019.02.046. |
| [141] |
FAN J, ZHOU J, WU ZQ, et al. Efficacy of different treatment strategies for hepatocellular carcinoma with portal vein tumor thrombosis[J]. World J Gastroenterol, 2005, 11(8): 1215-1219. DOI: 10.3748/wjg.v11.i8.1215. |
| [142] |
LO CM, LIU CL, CHAN SC, et al. A randomized, controlled trial of postoperative adjuvant interferon therapy after resection of hepatocellular carcinoma[J]. Ann Surg, 2007, 245(6): 831-842. DOI: 10.1097/01.sla.0000245829.00977.45. |
| [143] |
SUN HC, TANG ZY, WANG L, et al. Postoperative interferon alpha treatment postponed recurrence and improved overall survival in patients after curative resection of HBV-related hepatocellular carcinoma: A randomized clinical trial[J]. J Cancer Res Clin Oncol, 2006, 132(7): 458-465. DOI: 10.1007/s00432-006-0091-y. |
| [144] |
NISHIGUCHI S, TAMORI A, KUBO S. Effect of long-term postoperative interferon therapy on intrahepatic recurrence and survival rate after resection of hepatitis C virus-related hepatocellular carcinoma[J]. Intervirology, 2005, 48(1): 71-75. DOI: 10.1159/000082098. |
| [145] |
MAZZAFERRO V, ROMITO R, SCHIAVO M, et al. Prevention of hepatocellular carcinoma recurrence with alpha-interferon after liver resection in HCV cirrhosis[J]. Hepatology, 2006, 44(6): 1543-1554. DOI: 10.1002/hep.21415. |
| [146] |
JI J, SHI J, BUDHU A, et al. MicroRNA expression, survival, and response to interferon in liver cancer[J]. N Engl J Med, 2009, 361(15): 1437-1447. DOI: 10.1056/NEJMoa0901282. |
| [147] |
SUN HC, ZHU XD, ZHOU J, et al. Effect of postoperative apatinib treatment after resection of hepatocellular carcinoma with portal vein invasion: A phase Ⅱ study[J]. J Clin Oncol, 2020, 38(4_suppl): abstr 514. DOI: 10.1200/JCO.2020.38.4_suppl.514 |
| [148] |
SAPISOCHIN G, BRUIX J. Liver transplantation for hepatocellular carcinoma: Outcomes and novel surgical approaches[J]. Nat Rev Gastroenterol Hepatol, 2017, 14(4): 203-217. DOI: 10.1038/nrgastro.2016.193. |
| [149] |
FAN J, YANG GS, FU ZR, et al. Liver transplantation outcomes in 1, 078 hepatocellular carcinoma patients: A multi-center experience in Shanghai, China[J]. J Cancer Res Clin Oncol, 2009, 135(10): 1403-1412. DOI: 10.1007/s00432-009-0584-6. |
| [150] |
ZHENG SS, XU X, WU J, et al. Liver transplantation for hepatocellular carcinoma: Hangzhou experiences[J]. Transplantation, 2008, 85(12): 1726-1732. DOI: 10.1097/TP.0b013e31816b67e4. |
| [151] |
LI J, YAN LN, YANG J, et al. Indicators of prognosis after liver transplantation in Chinese hepatocellular carcinoma patients[J]. World J Gastroenterol, 2009, 15(33): 4170-4176. DOI: 10.3748/wjg.15.4170. |
| [152] |
SHAO Z, YANG GS, YANG N, et al. Application of Sanya Criteria in the treatment of liver transplantation for hepatocellular carcinoma[J]. Chin J Pract Surg, 2008, 28(6): 466-469. DOI: 10.3321/j.issn:1005-2208.2008.06.018. |
| [153] |
KULIK L, HEIMBACH JK, ZAIEM F, et al. Therapies for patients with hepatocellular carcinoma awaiting liver transplantation: A systematic review and meta-analysis[J]. Hepatology, 2018, 67(1): 381-400. DOI: 10.1002/hep.29485. |
| [154] |
LEE S, KIM KW, SONG GW, et al. The real impact of bridging or downstaging on survival outcomes after liver transplantation for hepatocellular carcinoma[J]. Liver Cancer, 2020, 9(6): 721-733. DOI: 10.1159/000507887. |
| [155] |
MAZZAFERRO V, CITTERIO D, BHOORI S, et al. Liver transplantation in hepatocellular carcinoma after tumour downstaging (XXL): A randomised, controlled, phase 2b/3 trial[J]. Lancet Oncol, 2020, 21(7): 947-956. DOI: 10.1016/S1470-2045(20)30224-2. |
| [156] |
MEHTA N, GUY J, FRENETTE CT, et al. Excellent outcomes of liver transplantation following down-staging of hepatocellular carcinoma to within Milan criteria: A multicenter study[J]. Clin Gastroenterol Hepatol, 2018, 16(6): 955-964. DOI: 10.1016/j.cgh.2017.11.037. |
| [157] |
LLOVET JM, PAVEL M, RIMOLA J, et al. Pilot study of living donor liver transplantation for patients with hepatocellular carcinoma exceeding Milan Criteria (Barcelona Clinic Liver Cancer extended criteria)[J]. Liver Transpl, 2018, 24(3): 369-379. DOI: 10.1002/lt.24977. |
| [158] |
PINHEIRO RS, WAISBERG DR, NACIF LS, et al. Living donor liver transplantation for hepatocellular cancer: An (almost) exclusive Eastern procedure?[J]. Transl Gastroenterol Hepatol, 2017, 2: 68. DOI: 10.21037/tgh.2017.08.02. |
| [159] |
SPOSITO C, CUCCHETTI A, MAZZAFERRO V. Assessing competing risks for death following liver transplantation for hepatocellular carcinoma[J]. Dig Dis Sci, 2019, 64(4): 1001-1007. DOI: 10.1007/s10620-019-05538-1. |
| [160] |
SEGEV DL, SOZIO SM, SHIN EJ, et al. Steroid avoidance in liver transplantation: Meta-analysis and meta-regression of randomized trials[J]. Liver Transpl, 2008, 14(4): 512-525. DOI: 10.1002/lt.21396. |
| [161] |
RODRÍGUEZ-PERÁLVAREZ M, TSOCHATZIS E, NAVEAS MC, et al. Reduced exposure to calcineurin inhibitors early after liver transplantation prevents recurrence of hepatocellular carcinoma[J]. J Hepatol, 2013, 59(6): 1193-1199. DOI: 10.1016/j.jhep.2013.07.012. |
| [162] |
LIANG W, WANG D, LING X, et al. Sirolimus-based immunosuppression in liver transplantation for hepatocellular carcinoma: A meta-analysis[J]. Liver Transpl, 2012, 18(1): 62-69. DOI: 10.1002/lt.22441. |
| [163] |
ZHOU J, WANG Z, WU ZQ, et al. Sirolimus-based immunosuppression therapy in liver transplantation for patients with hepatocellular carcinoma exceeding the Milan criteria[J]. Transplant Proc, 2008, 40(10): 3548-3553. DOI: 10.1016/j.transproceed.2008.03.165. |
| [164] |
GEISSLER EK, SCHNITZBAUER AA, ZVLKE C, et al. Sirolimus use in liver transplant recipients with hepatocellular carcinoma: A randomized, multicenter, open-label phase 3 trial[J]. Transplantation, 2016, 100(1): 116-125. DOI: 10.1097/TP.0000000000000965. |
| [165] |
THORAT A, JENG LB, YANG HR, et al. Assessing the role of everolimus in reducing hepatocellular carcinoma recurrence after living donor liver transplantation for patients within the UCSF criteria: Re-inventing the role of mammalian target of rapamycin inhibitors[J]. Ann Hepatobiliary Pancreat Surg, 2017, 21(4): 205-211. DOI: 10.14701/ahbps.2017.21.4.205. |
| [166] |
SCHNITZBAUER AA, FILMANN N, ADAM R, et al. mTOR inhibition is most beneficial after liver transplantation for hepatocellular carcinoma in patients with active tumors[J]. Ann Surg, 2020, 272(5): 855-862. DOI: 10.1097/SLA.0000000000004280. |
| [167] |
FILGUEIRA NA. Hepatocellular carcinoma recurrence after liver transplantation: Risk factors, screening and clinical presentation[J]. World J Hepatol, 2019, 11(3): 261-272. DOI: 10.4254/wjh.v11.i3.261. |
| [168] |
AU KP, CHOK KSH. Multidisciplinary approach for post-liver transplant recurrence of hepatocellular carcinoma: A proposed management algorithm[J]. World J Gastroenterol, 2018, 24(45): 5081-5094. DOI: 10.3748/wjg.v24.i45.5081. |
| [169] |
IAVARONE M, INVERNIZZI F, CZAUDERNA C, et al. Preliminary experience on safety of regorafenib after sorafenib failure in recurrent hepatocellular carcinoma after liver transplantation[J]. Am J Transplant, 2019, 19(11): 3176-3184. DOI: 10.1111/ajt.15551. |
| [170] |
NORDNESS MF, HAMEL S, GODFREY CM, et al. Fatal hepatic necrosis after nivolumab as a bridge to liver transplant for HCC: Are checkpoint inhibitors safe for the pretransplant patient?[J]. Am J Transplant, 2020, 20(3): 879-883. DOI: 10.1111/ajt.15617. |
| [171] |
SHI GM, WANG J, HUANG XW, et al. Graft programmed death ligand 1 expression as a marker for transplant rejection following anti-programmed death 1 immunotherapy for recurrent liver tumors[J]. Liver Transpl, 2021, 27(3): 444-449. DOI: 10.1002/lt.25887. |
| [172] |
HASEGAWA K, AOKI T, ISHIZAWA T, et al. Comparison of the therapeutic outcomes between surgical resection and percutaneous ablation for small hepatocellular carcinoma[J]. Ann Surg Oncol, 2014, 21(Suppl 3): S348-S355. DOI: 10.1245/s10434-014-3585-x. |
| [173] |
LI L, ZHANG J, LIU X, et al. Clinical outcomes of radiofrequency ablation and surgical resection for small hepatocellular carcinoma: A meta-analysis[J]. J Gastroenterol Hepatol, 2012, 27(1): 51-58. DOI: 10.1111/j.1440-1746.2011.06947.x. |
| [174] |
HUANG J, YAN L, CHENG Z, et al. A randomized trial comparing radiofrequency ablation and surgical resection for HCC conforming to the Milan criteria[J]. Ann Surg, 2010, 252(6): 903-912. DOI: 10.1097/SLA.0b013e3181efc656. |
| [175] |
FENG Q, CHI Y, LIU Y, et al. Efficacy and safety of percutaneous radiofrequency ablation versus surgical resection for small hepatocellular carcinoma: A meta-analysis of 23 studies[J]. J Cancer Res Clin Oncol, 2015, 141(1): 1-9. DOI: 10.1007/s00432-014-1708-1. |
| [176] |
CHEN QW, YING HF, GAO S, et al. Radiofrequency ablation plus chemoembolization versus radiofrequency ablation alone for hepatocellular carcinoma: A systematic review and meta-analysis[J]. Clin Res Hepatol Gastroenterol, 2016, 40(3): 309-314. DOI: 10.1016/j.clinre.2015.07.008. |
| [177] |
MORIMOTO M, NUMATA K, KONDOU M, et al. Midterm outcomes in patients with intermediate-sized hepatocellular carcinoma: A randomized controlled trial for determining the efficacy of radiofrequency ablation combined with transcatheter arterial chemoembolization[J]. Cancer, 2010, 116(23): 5452-5460. DOI: 10.1002/cncr.25314. |
| [178] |
PENG ZW, ZHANG YJ, CHEN MS, et al. Radiofrequency ablation with or without transcatheter arterial chemoembolization in the treatment of hepatocellular carcinoma: A prospective randomized trial[J]. J Clin Oncol, 2013, 31(4): 426-432. DOI: 10.1200/JCO.2012.42.9936. |
| [179] |
WANG L, KE Q, LIN N, et al. The efficacy of transarterial chemoembolization combined with microwave ablation for unresectable hepatocellular carcinoma: A systematic review and meta-analysis[J]. Int J Hyperthermia, 2019, 36(1): 1288-1296. DOI: 10.1080/02656736.2019.1692148. |
| [180] |
LIVRAGHI T, MELONI F, DI STASI M, et al. Sustained complete response and complications rates after radiofrequency ablation of very early hepatocellular carcinoma in cirrhosis: Is resection still the treatment of choice?[J]. Hepatology, 2008, 47(1): 82-89. DOI: 10.1002/hep.21933. |
| [181] |
PENG ZW, LIN XJ, ZHANG YJ, et al. Radiofrequency ablation versus hepatic resection for the treatment of hepatocellular carcinomas 2 cm or smaller: A retrospective comparative study[J]. Radiology, 2012, 262(3): 1022-1033. DOI: 10.1148/radiol.11110817. |
| [182] |
VIETTI VIOLI N, DURAN R, GUIU B, et al. Efficacy of microwave ablation versus radiofrequency ablation for the treatment of hepatocellular carcinoma in patients with chronic liver disease: A randomised controlled phase 2 trial[J]. Lancet Gastroenterol Hepatol, 2018, 3(5): 317-325. DOI: 10.1016/S2468-1253(18)30029-3. |
| [183] |
YU J, YU XL, HAN ZY, et al. Percutaneous cooled-probe microwave versus radiofrequency ablation in early-stage hepatocellular carcinoma: A phase Ⅲ randomised controlled trial[J]. Gut, 2017, 66(6): 1172-1173. DOI: 10.1136/gutjnl-2016-312629. |
| [184] |
TAN W, DENG Q, LIN S, et al. Comparison of microwave ablation and radiofrequency ablation for hepatocellular carcinoma: A systematic review and meta-analysis[J]. Int J Hyperthermia, 2019, 36(1): 264-272. DOI: 10.1080/02656736.2018.1562571. |
| [185] |
DI VECE F, TOMBESI P, ERMILI F, et al. Coagulation areas produced by cool-tip radiofrequency ablation and microwave ablation using a device to decrease back-heating effects: A prospective pilot study[J]. Cardiovasc Intervent Radiol, 2014, 37(3): 723-729. DOI: 10.1007/s00270-013-0733-9. |
| [186] |
LIN SM, LIN CJ, LIN CC, et al. Randomised controlled trial comparing percutaneous radiofrequency thermal ablation, percutaneous ethanol injection, and percutaneous acetic acid injection to treat hepatocellular carcinoma of 3 cm or less[J]. Gut, 2005, 54(8): 1151-1156. DOI: 10.1136/gut.2004.045203. |
| [187] |
AHMED M, SOLBIATI L, BRACE CL, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria—a 10-year update[J]. Radiology, 2014, 273(1): 241-260. DOI: 10.1148/radiol.14132958. |
| [188] |
LI L, WANG W, PAN H, et al. Microwave ablation combined with OK-432 induces Th1-type response and specific antitumor immunity in a murine model of breast cancer[J]. J Transl Med, 2017, 15(1): 23. DOI: 10.1186/s12967-017-1124-9. |
| [189] |
MIZUKOSHI E, YAMASHITA T, ARAI K, et al. Enhancement of tumor-associated antigen-specific T cell responses by radiofrequency ablation of hepatocellular carcinoma[J]. Hepatology, 2013, 57(4): 1448-1457. DOI: 10.1002/hep.26153. |
| [190] |
SLOVAK R, LUDWIG JM, GETTINGER SN, et al. Immuno-thermal ablations - boosting the anticancer immune response[J]. J Immunother Cancer, 2017, 5(1): 78. DOI: 10.1186/s40425-017-0284-8. |
| [191] |
DUAN X, WANG M, HAN X, et al. Combined use of microwave ablation and cell immunotherapy induces nonspecific immunity of hepatocellular carcinoma model mice[J]. Cell Cycle, 2020, 19(24): 3595-3607. DOI: 10.1080/15384101.2020.1853942. |
| [192] |
ROZEMAN EA, PREVOO W, MEIER M, et al. Phase Ⅰb/Ⅱ trial testing combined radiofrequency ablation and ipilimumab in uveal melanoma (SECIRA-UM)[J]. Melanoma Res, 2020, 30(3): 252-260. DOI: 10.1097/CMR.0000000000000653. |
| [193] |
LENCIONI R, DE BAERE T, SOULEN MC, et al. Lipiodol transarterial chemoembolization for hepatocellular carcinoma: A systematic review of efficacy and safety data[J]. Hepatology, 2016, 64(1): 106-116. DOI: 10.1002/hep.28453. |
| [194] |
PELLETIER G, DUCREUX M, GAY F, et al. Treatment of unresectable hepatocellular carcinoma with lipiodol chemoembolization: A multicenter randomized trial. Groupe CHC[J]. J Hepatol, 1998, 29(1): 129-134. DOI: 10.1016/s0168-8278(98)80187-6. |
| [195] |
LO CM, NGAN H, TSO WK, et al. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma[J]. Hepatology, 2002, 35(5): 1164-1171. DOI: 10.1053/jhep.2002.33156. |
| [196] |
LLOVET JM, REAL MI, MONTAÑA X, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: A randomised controlled trial[J]. Lancet, 2002, 359(9319): 1734-1739. DOI: 10.1016/S0140-6736(02)08649-X. |
| [197] |
CAMMÀ C, SCHEPIS F, ORLANDO A, et al. Transarterial chemoembolization for unresectable hepatocellular carcinoma: Meta-analysis of randomized controlled trials[J]. Radiology, 2002, 224(1): 47-54. DOI: 10.1148/radiol.2241011262. |
| [198] |
LLOVET JM, BRUIX J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: Chemoembolization improves survival[J]. Hepatology, 2003, 37(2): 429-442. DOI: 10.1053/jhep.2003.50047. |
| [199] |
Cooperative Group of Interventional Group, Chinese Society of Radiology, Chinese Medical Association. Expert consensus on technical operational standard of transcatheter arterial chemoembolization for primary hepatocellular carcinoma[J]. Chin J Radiol, 2011, 45(10): 908-912. DOI: 10.3760/cma.j.issn.1005-1201.2011.10.003. |
| [200] |
Clinical Guidelines Committee of Chinese Interventionalists College. Chinese clinical practice guidelines for transarterial chemoembolization of hepatocellular carcinoma[J]. Natl Med J China, 2021, 101(24): 1848-1862. DOI: 10.3760/cma.j.cn112137-20210425-00991. |
| [201] |
Tumor Intervention Expert Committee of Chinese Anti—Cancer Associcaion. Chinese tumor intervention expert consensus on the application principles of transcatheter arterial infusion chemotherapy[J]. J Interv Radiol, 2017, 26(11): 963-970. DOI: 10.3969/j.issn.1008-794X.2017.11.001. |
| [202] |
GUO Z, TENG GJ, ZOU YH, et al. Transarterial treatment of primary and secondary liver cancer with drug-eluting beads transarterial chemoembolization: Technical recommendations[J]. Chin J Radiol, 2019, 53(5): 336-340. DOI: 10.3760/cma.j.issn.1005-1201.2019.05.002. |
| [203] |
MIYAYAMA S, MATSUI O. Superselective conventional transarterial chemoembolization for hepatocellular carcinoma: Rationale, technique, and outcome[J]. J Vasc Interv Radiol, 2016, 27(9): 1269-1278. DOI: 10.1016/j.jvir.2016.04.014. |
| [204] |
PUNG L, AHMAD M, MUELLER K, et al. The role of cone-beam CT in transcatheter arterial chemoembolization for hepatocellular carcinoma: A systematic review and meta-analysis[J]. J Vasc Interv Radiol, 2017, 28(3): 334-341. DOI: 10.1016/j.jvir.2016.11.037. |
| [205] |
LIANG B, MAKAMURE J, SHU S, et al. Treatment response, survival, and safety of transarterial chemoembolization with callispheres(®) microspheres versus conventional transarterial chemoembolization in hepatocellular carcinoma: A Meta-analysis[J]. Front Oncol, 2021, 11: 576232. DOI: 10.3389/fonc.2021.576232. |
| [206] |
SI ZM, WANG GZ, QIAN S, et al. Combination therapies in the management of large (≥ 5 cm) hepatocellular carcinoma: microwave ablation immediately followed by transarterial chemoembolization[J]. J Vasc Interv Radiol, 2016, 27(10): 1577-1583. DOI: 10.1016/j.jvir.2016.02.014. |
| [207] |
LEWIS AR, PADULA CA, MCKINNEY JM, et al. Ablation plus transarterial embolic therapy for hepatocellular carcinoma larger than 3 cm: Science, evidence, and future directions[J]. Semin Intervent Radiol, 2019, 36(4): 303-309. DOI: 10.1055/s-0039-1697641. |
| [208] |
HUO YR, ESLICK GD. Transcatheter arterial chemoembolization plus radiotherapy compared with chemoembolization alone for hepatocellular carcinoma: A systematic review and Meta-analysis[J]. JAMA Oncol, 2015, 1(6): 756-765. DOI: 10.1001/jamaoncol.2015.2189. |
| [209] |
Radiation Oncology Branch of the Chinese Medical Association, Expert Committee on Liver Cancer and Digestive System of China Institute of Biomedical Engineering, Liver Cancer Research Group of Radiation Oncology Branch of China Research Hospital. Consensus on radiation therapy for primary liver cancer in 2016[J]. Chin J Radiat Oncol, 2016, 25(11): 1141-1150. DOI: 10.3760/cma.j.issn.1004-4221.2016.11.001. |
| [210] |
JANG JW, CHOI JY, BAE SH, et al. Transarterial chemo-lipiodolization can reactivate hepatitis B virus replication in patients with hepatocellular carcinoma[J]. J Hepatol, 2004, 41(3): 427-435. DOI: 10.1016/j.jhep.2004.05.014. |
| [211] |
Chinese Society of Infectious Diseases, Chinese Medical Association, Chinese Society of Hepatology, Chinese Medical Association. Guidelines for the prevention and treatment of chronic hepatitis B (version 2019)[J]. J Clin Hepatol, 2019, 35(12): 2648-2669. DOI: 10.3969/j.issn.1001-5256.2019.12.007. |
| [212] |
YANG M, FANG Z, YAN Z, et al. Transarterial chemoembolisation (TACE) combined with endovascular implantation of an iodine-125 seed strand for the treatment of hepatocellular carcinoma with portal vein tumour thrombosis versus TACE alone: A two-arm, randomised clinical trial[J]. J Cancer Res Clin Oncol, 2014, 140(2): 211-219. DOI: 10.1007/s00432-013-1568-0. |
| [213] |
HU HT, LI HL, GUO CY, et al. Transcatheter arterial chemoembolization combined 125iodine seed implantation for primary hepatic carcinoma with portal vein tumor thrombus thrombosis[J]. Chin J Radiol, 2012, 46(6): 552-556. DOI: 10.3760/cma.j.issn.1005-1201.2012.06.016. |
| [214] |
ZHANG ZH, ZHANG W, GU JY, et al. Treatment of hepatocellular carcinoma with tumor thrombus with the use of iodine-125 seed strand implantation and transarterial chemoembolization: A propensity-score analysis[J]. J Vasc Interv Radiol, 2018, 29(8): 1085-1093. DOI: 10.1016/j.jvir.2018.02.013. |
| [215] |
CHEN YX, ZHUANG Y, YANG P, et al. Helical IMRT-based stereotactic body radiation therapy using an abdominal compression technique and modified fractionation regimen for small hepatocellular carcinoma[J]. Technol Cancer Res Treat, 2020, 19: 1533033820937002. DOI: 10.1177/1533033820937002. |
| [216] |
CHINO F, STEPHENS SJ, CHOI SS, et al. The role of external beam radiotherapy in the treatment of hepatocellular cancer[J]. Cancer, 2018, 124(17): 3476-3489. DOI: 10.1002/cncr.31334. |
| [217] |
HARA K, TAKEDA A, TSURUGAI Y, et al. Radiotherapy for hepatocellular carcinoma results in comparable survival to radiofrequency ablation: A propensity score analysis[J]. Hepatology, 2019, 69(6): 2533-2545. DOI: 10.1002/hep.30591. |
| [218] |
JANG WI, BAE SH, KIM MS, et al. A phase 2 multicenter study of stereotactic body radiotherapy for hepatocellular carcinoma: Safety and efficacy[J]. Cancer, 2020, 126(2): 363-372. DOI: 10.1002/cncr.32502. |
| [219] |
KIM N, CHENG J, JUNG I, et al. Stereotactic body radiation therapy vs. radiofrequency ablation in Asian patients with hepatocellular carcinoma[J]. J Hepatol, 2020, 73(1): 121-129. DOI: 10.1016/j.jhep.2020.03.005. |
| [220] |
SU TS, LIANG P, LIANG J, et al. Long-term survival analysis of stereotactic ablative radiotherapy versus liver resection for small hepatocellular carcinoma[J]. Int J Radiat Oncol Biol Phys, 2017, 98(3): 639-646. DOI: 10.1016/j.ijrobp.2017.02.095. |
| [221] |
WAHL DR, STENMARK MH, TAO Y, et al. Outcomes after stereotactic body radiotherapy or radiofrequency ablation for hepatocellular carcinoma[J]. J Clin Oncol, 2016, 34(5): 452-459. DOI: 10.1200/JCO.2015.61.4925. |
| [222] |
MENG MB, CUI YL, LU Y, et al. Transcatheter arterial chemoembolization in combination with radiotherapy for unresectable hepatocellular carcinoma: A systematic review and meta-analysis[J]. Radiother Oncol, 2009, 92(2): 184-194. DOI: 10.1016/j.radonc.2008.11.002. |
| [223] |
OHRI N, DAWSON LA, KRISHNAN S, et al. Radiotherapy for hepatocellular carcinoma: New indications and directions for future study[J]. J Natl Cancer Inst, 2016, 108(9): djw133. DOI: 10.1093/jnci/djw133. |
| [224] |
YOON SM, RYOO BY, LEE SJ, et al. Efficacy and safety of transarterial chemoembolization plus external beam radiotherapy vs sorafenib in hepatocellular carcinoma with macroscopic vascular invasion: A randomized clinical trial[J]. JAMA Oncol, 2018, 4(5): 661-669. DOI: 10.1001/jamaoncol.2017.5847. |
| [225] |
ZENG ZC, FAN J, TANG ZY, et al. A comparison of treatment combinations with and without radiotherapy for hepatocellular carcinoma with portal vein and/or inferior vena cava tumor thrombus[J]. Int J Radiat Oncol Biol Phys, 2005, 61(2): 432-443. DOI: 10.1016/j.ijrobp.2004.05.025. |
| [226] |
SHEN L, XI M, ZHAO L, et al. Combination therapy after TACE for hepatocellular carcinoma with macroscopic vascular invasion: Stereotactic body radiotherapy versus Sorafenib[J]. Cancers (Basel), 2018, 10(12): 516. DOI: 10.3390/cancers10120516. |
| [227] |
SUN J, YANG L, SHI J, et al. Postoperative adjuvant IMRT for patients with HCC and portal vein tumor thrombus: An open-label randomized controlled trial[J]. Radiother Oncol, 2019, 140: 20-25. DOI: 10.1016/j.radonc.2019.05.006. |
| [228] |
JIHYE C, JINSIL S. Application of radiotherapeutic strategies in the BCLC-defined stages of hepatocellular carcinoma[J]. Liver Cancer, 2012, 1(3-4): 216-225. DOI: 10.1159/000343836. |
| [229] |
SOLIMAN H, RINGASH J, JIANG H, et al. Phase Ⅱ trial of palliative radiotherapy for hepatocellular carcinoma and liver metastases[J]. J Clin Oncol, 2013, 31(31): 3980-3986. DOI: 10.1200/JCO.2013.49.9202. |
| [230] |
SAPISOCHIN G, BARRY A, DOHERTY M, et al. Stereotactic body radiotherapy vs TACE or RFA as a bridge to transplant in patients with hepatocellular carcinoma. An intention-to-treat analysis[J]. J Hepatol, 2017, 67(1): 92-99. DOI: 10.1016/j.jhep.2017.02.022. |
| [231] |
WANG WH, WANG Z, WU JX, et al. Survival benefit with IMRT following narrow-margin hepatectomy in patients with hepatocellular carcinoma close to major vessels[J]. Liver Int, 2015, 35(12): 2603-2610. DOI: 10.1111/liv.12857. |
| [232] |
WANG L, WANG W, RONG W, et al. Postoperative adjuvant treatment strategy for hepatocellular carcinoma with microvascular invasion: A non-randomized interventional clinical study[J]. BMC Cancer, 2020, 20(1): 614. DOI: 10.1186/s12885-020-07087-7. |
| [233] |
BUJOLD A, MASSEY CA, KIM JJ, et al. Sequential phase Ⅰ and Ⅱ trials of stereotactic body radiotherapy for locally advanced hepatocellular carcinoma[J]. J Clin Oncol, 2013, 31(13): 1631-1639. DOI: 10.1200/JCO.2012.44.1659. |
| [234] |
|
| [235] |
HE J, SHI S, YE L, et al. A randomized trial of conventional fraction versus hypofraction radiotherapy for bone metastases from hepatocellular carcinoma[J]. J Cancer, 2019, 10(17): 4031-4037. DOI: 10.7150/jca.28674. |
| [236] |
HOU JZ, ZENG ZC, WANG BL, et al. High dose radiotherapy with image-guided hypo-IMRT for hepatocellular carcinoma with portal vein and/or inferior vena cava tumor thrombi is more feasible and efficacious than conventional 3D-CRT[J]. Jpn J Clin Oncol, 2016, 46(4): 357-362. DOI: 10.1093/jjco/hyv205. |
| [237] |
ZHANG H, CHEN Y, HU Y, et al. Image-guided intensity-modulated radiotherapy improves short-term survival for abdominal lymph node metastases from hepatocellular carcinoma[J]. Ann Palliat Med, 2019, 8(5): 717-727. DOI: 10.21037/apm.2019.11.17. |
| [238] |
BYUN HK, KIM HJ, IM YR, et al. Dose escalation in radiotherapy for incomplete transarterial chemoembolization of hepatocellular carcinoma[J]. Strahlenther Onkol, 2020, 196(2): 132-141. DOI: 10.1007/s00066-019-01488-9. |
| [239] |
HU Y, ZHOU YK, CHEN YX, et al. 4D-CT scans reveal reduced magnitude of respiratory liver motion achieved by different abdominal compression plate positions in patients with intrahepatic tumors undergoing helical tomotherapy[J]. Med Phys, 2016, 43(7): 4335. DOI: 10.1118/1.4953190. |
| [240] |
KIM TH, KOH YH, KIM BH, et al. Proton beam radiotherapy vs. radiofrequency ablation for recurrent hepatocellular carcinoma: A randomized phase Ⅲ trial[J]. J Hepatol, 2021, 74(3): 603-612. DOI: 10.1016/j.jhep.2020.09.026. |
| [241] |
BIAN H, ZHENG JS, NAN G, et al. Randomized trial of[131I] metuximab in treatment of hepatocellular carcinoma after percutaneous radiofrequency ablation[J]. J Natl Cancer Inst, 2014, 106(9) : dju239. DOI: 10.1093/jnci/dju239. |
| [242] |
Working Committee for Treatment of Bone Metastasis, Chinese Society of Nuclear Medicine, Chinese Medical Association. Expert consensus on strontium-89 chloride treatment of bone metastases (2017)[J]. Chin J Nuclear Med Mol Imaging, 2018, 38(6): 412-415. DOI: 10.3760/cma.j.issn.2095-2848.2018.06.008. |
| [243] |
FINN RS, QIN S, IKEDA M, et al. Atezolizumab plus Bevacizumab in unresectable hepatocellular carcinoma[J]. N Engl J Med, 2020, 382(20): 1894-1905. DOI: 10.1056/NEJMoa1915745. |
| [244] |
FINN RS, QIN S, IKEDA M, et al. IMbrave150: Updated overall survival (OS) data from a global, randomized, open-label phase Ⅲ study of atezolizumab (atezo) + bevacizumab (bev) versus sorafenib (sor) in patients (pts) with unresectable hepatocellular carcinoma (HCC)[J]. J Clin Oncol, 2021, 39(suppl 3): 267.
|
| [245] |
REN Z, XU J, BAI Y, et al. Sintilimab plus a bevacizumab biosimilar (IBI305) versus sorafenib in unresectable hepatocellular carcinoma (ORIENT-32): A randomised, open-label, phase 2-3 study[J]. Lancet Oncol, 2021, 22(7): 977-990. DOI: 10.1016/S1470-2045(21)00252-7. |
| [246] |
QIN S, BI F, GU S, et al. Donafenib versus sorafenib in first-line treatment of unresectable or metastatic hepatocellular carcinoma: A randomized, open-label, parallel-controlled phase Ⅱ-Ⅲ trial[J]. J Clin Oncol, 2021, 39(27): 3002-3011. DOI: 10.1200/JCO.21.00163. |
| [247] |
KUDO M, FINN RS, QIN S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: A randomised phase 3 non-inferiority trial[J]. Lancet, 2018, 391(10126): 1163-1173. DOI: 10.1016/S0140-6736(18)30207-1. |
| [248] |
LLOVET JM, RICCI S, MAZZAFERRO V, et al. Sorafenib in advanced hepatocellular carcinoma[J]. N Engl J Med, 2008, 359(4): 378-390. DOI: 10.1056/NEJMoa0708857. |
| [249] |
CHENG AL, KANG YK, CHEN Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: A phase Ⅲ randomised, double-blind, placebo-controlled trial[J]. Lancet Oncol, 2009, 10(1): 25-34. DOI: 10.1016/S1470-2045(08)70285-7. |
| [250] |
PRESSIANI T, BONI C, RIMASSA L, et al. Sorafenib in patients with Child-Pugh class A and B advanced hepatocellular carcinoma: A prospective feasibility analysis[J]. Ann Oncol, 2013, 24(2): 406-411. DOI: 10.1093/annonc/mds343. |
| [251] |
QIN S, BAI Y, LIM HY, et al. Randomized, multicenter, open-label study of oxaliplatin plus fluorouracil/leucovorin versus doxorubicin as palliative chemotherapy in patients with advanced hepatocellular carcinoma from Asia[J]. J Clin Oncol, 2013, 31(28): 3501-3508. DOI: 10.1200/JCO.2012.44.5643. |
| [252] |
QIN S, CHENG Y, LIANG J, et al. Efficacy and safety of the FOLFOX4 regimen versus doxorubicin in Chinese patients with advanced hepatocellular carcinoma: A subgroup analysis of the EACH study[J]. Oncologist, 2014, 19(11): 1169-1178. DOI: 10.1634/theoncologist.2014-0190. |
| [253] |
QU FL, HAO XZ, QIN SK, et al. Multicenter phase Ⅱ clinical trial of arsenic trioxide injection in the treatment of primary hepatocarcinoma[J]. Chin J Oncol, 2011, 33(9): 697-701. DOI: 10.3760/cma.j.issn.0253-3766.2011.09.013. |
| [254] |
BRUIX J, QIN S, MERLE P, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): A randomised, double-blind, placebo-controlled, phase 3 trial[J]. Lancet, 2017, 389(10064): 56-66. DOI: 10.1016/S0140-6736(16)32453-9. |
| [255] |
QIN S, LI Q, GU S, et al. Apatinib as second-line or later therapy in patients with advanced hepatocellular carcinoma (AHELP): A multicentre, double-blind, randomised, placebo-controlled, phase 3 trial[J]. Lancet Gastroenterol Hepatol, 2021, 6(7): 559-568. DOI: 10.1016/S2468-1253(21)00109-6. |
| [256] |
QIN S, REN Z, MENG Z, et al. Camrelizumab in patients with previously treated advanced hepatocellular carcinoma: A multicentre, open-label, parallel-group, randomised, phase 2 trial[J]. Lancet Oncol, 2020, 21(4): 571-580. DOI: 10.1016/S1470-2045(20)30011-5. |
| [257] |
XU J, SHEN J, GU S, et al. Camrelizumab in combination with apatinib in patients with advanced hepatocellular carcinoma (RESCUE): A nonrandomized, open-label, phase Ⅱ trial[J]. Clin Cancer Res, 2021, 27(4): 1003-1011. DOI: 10.1158/1078-0432.CCR-20-2571. |
| [258] |
XU J, ZHANG Y, JIA R, et al. Anti-PD-1 antibody SHR-1210 combined with apatinib for advanced hepatocellular carcinoma, gastric, or esophagogastric junction cancer: An open-label, dose escalation and expansion study[J]. Clin Cancer Res, 2019, 25(2): 515-523. DOI: 10.1158/1078-0432.CCR-18-2484. |
| [259] |
DUCREUX M, ABOU-ALFA GK, REN Z, et al. Results from a global Phase 2 study of tislelizumab, an investigational PD-1 antibody, in patients with previously treated advanced hepatocellular carcinoma[C]. ESMO WCGI, 2021.
|
| [260] |
ZHU AX, FINN RS, EDELINE J, et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): A non-randomised, open-label phase 2 trial[J]. Lancet Oncol, 2018, 19(7): 940-952. DOI: 10.1016/S1470-2045(18)30351-6. |
| [261] |
YAU T, KANG YK, KIM TY, et al. Efficacy and safety of nivolumab plus ipilimumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib: The checkmate 040 randomized clinical trial[J]. JAMA Oncol, 2020, 6(11): e204564. DOI: 10.1001/jamaoncol.2020.4564. |
| [262] |
ABOU-ALFA GK, MEYER T, CHENG AL, et al. Cabozantinib in patients with advanced and progressing hepatocellular carcinoma[J]. N Engl J Med, 2018, 379(1): 54-63. DOI: 10.1056/NEJMoa1717002. |
| [263] |
ZHU AX, PARK JO, RYOO BY, et al. Ramucirumab versus placebo as second-line treatment in patients with advanced hepatocellular carcinoma following first-line therapy with sorafenib (REACH): A randomised, double-blind, multicentre, phase 3 trial[J]. Lancet Oncol, 2015, 16(7): 859-870. DOI: 10.1016/S1470-2045(15)00050-9. |
| [264] |
ZHU AX, KANG YK, YEN CJ, et al. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased α-fetoprotein concentrations (REACH-2): A randomised, double-blind, placebo-controlled, phase 3 trial[J]. Lancet Oncol, 2019, 20(2): 282-296. DOI: 10.1016/S1470-2045(18)30937-9. |
| [265] |
CAI DF. Establishment of an integrated traditional Chinese and Western medicine system based on disease and syndrome differentiation[J]. Chin J Integr Trad West Med, 2019, 39(9): 1034-1035. DOI: 10.7661/j.cjim.20190114.040. |
| [266] |
CAI DF. An analysis of the clinical diagnosis and treatment mode combining disease and syndrome[J]. Chin J Integr Trad West Med, 2019, 39(2): 133-135. DOI: 10.7661/j.cjim.20190114.040. |
| [267] |
ZHAI XF, LIU XL, SHEN F, et al. Traditional herbal medicine prevents postoperative recurrence of small hepatocellular carcinoma: A randomized controlled study[J]. Cancer, 2018, 124(10): 2161-2168. DOI: 10.1002/cncr.30915. |
| [268] |
QIN SK, LI Q, MING XU J, et al. Icaritin-induced immunomodulatory efficacy in advanced hepatitis B virus-related hepatocellular carcinoma: Immunodynamic biomarkers and overall survival[J]. Cancer Sci, 2020, 111(11): 4218-4231. DOI: 10.1111/cas.14641. |
| [269] |
YU Z, GUO J, HU M, et al. Icaritin exacerbates mitophagy and synergizes with doxorubicin to induce immunogenic cell death in hepatocellular carcinoma[J]. ACS Nano, 2020, 14(4): 4816-4828. DOI: 10.1021/acsnano.0c00708. |
| [270] |
CAI WH, YIN CL, FAN QQ. Clinical effect of Aidi injection combined with transcatheter arterial chemoembolization in treatment of advanced primary liver cancer[J]. J Chin Physician, 2018, 20(11): 1723-1725. DOI: 10.3760/cma.j.issn.1008-1372.2018.11.032 |
| [271] |
CHENG Y, HUA HQ. Research progress on anti-hepatoma mechanisms and clinical application of β-elemene[J]. Chin Clin Oncol, 2017, 22(10): 950-953. DOI: 10.3969/j.issn.1009-0460.2017.10.018. |
| [272] |
FAN S, LI QY, ZHOU ZT, et al. Clinical effect of TACE combined with Jinlong capsules in treatment of primary liver cancer[J]. China Prac Med, 2019, 14(21): 42-44. DOI: 10.14163/j.cnki.11-5547/r.2019.21.022. |
| [273] |
GAO JL. Prospective randomized controlled study on advanced primary hepatic cancer treated by Ganfule prescription[J]. China J Chin Mater Med, 2014, 39(12): 2367-2369. DOI: 10.4268/cjcmm20141243. |
| [274] |
|
| [275] |
YANG XH. Clinical effect of TACE combined with intravenous dripping of Brucea javanica oil emulsion in patients with liver cancer and its influence on the level of vascular endothelial growth factor[J]. Strait Pharm J, 2017, 29(9): 176-177. DOI: 10.3969/j.issn.1006-3765.2017.09.090. |
| [276] |
European Association for the Study of the Liver. EASL recommendations on treatment of hepatitis C 2018[J]. J Hepatol, 2018, 69(2): 461-511. DOI: 10.1016/j.jhep.2018.03.026. |
| [277] |
Chinese Society of Hepatology, Chinese Medical Association, Chinese Society of Infectious Diseases, Chinese Medical Association. Guidelines for the prevention and treatment of hepatitis C (2019 version)[J]. J Clin Hepatol, 2019, 35(12): 2670-2686. DOI: 10.3969/j.issn.1001-5256.2019.12.008. |
| [278] |
Guidelines Committee of Chinese Society of Clinical Oncology. Chinese Society of Clinical Oncology (CSCO) guidelines for standardized management of tumor chemoradiotherapy-related neutropenia (Version 2021)[J]. 2021, 26(7): 638-648. DOI: 10.3969/j.issn.1009-0460.2021.07.011. |
| [279] |
The Society of Chemotherapy, China Anti-Cancer Association; Committee of Neoplastic Supportive-Care (CONS), China Anti-Cancer Association. Consensus on the clinical diagnosis, treatment, and prevention of chemotherapy-in-duced thrombocytopenia in China (2019 version)[J]. Chin J Clin Oncol, 2019, 46(18): 923-929. DOI: 10.3969/j.issn.1000-8179.2019.18.914. |
| [280] |
SEYMOUR L, BOGAERTS J, PERRONE A, et al. iRECIST: Guidelines for response criteria for use in trials testing immunotherapeutics[J]. Lancet Oncol, 2017, 18(3): e143-e152. DOI: 10.1016/S1470-2045(17)30074-8. |
| [281] |
MORIS D, CHAKEDIS J, SUN SH, et al. Management, outcomes, and prognostic factors of ruptured hepatocellular carcinoma: A systematic review[J]. J Surg Oncol, 2018, 117(3): 341-353. DOI: 10.1002/jso.24869. |
| [282] |
SAHU SK, CHAWLA YK, DHIMAN RK, et al. Rupture of hepatocellular carcinoma: A review of literature[J]. J Clin Exp Hepatol, 2019, 9(2): 245-256. DOI: 10.1016/j.jceh.2018.04.002. |
| [283] |
TAN NP, MAJEED A, ROBERTS SK, et al. Survival of patients with ruptured and non-ruptured hepatocellular carcinoma[J]. Med J Aust, 2020, 212(6): 277-278. DOI: 10.5694/mja2.50483. |
| [284] |
YOSHIDA H, MAMADA Y, TANIAI N, et al. Spontaneous ruptured hepatocellular carcinoma[J]. Hepatol Res, 2016, 46(1): 13-21. DOI: 10.1111/hepr.12498. |
| [285] |
ZHONG F, CHENG XS, HE K, et al. Treatment outcomes of spontaneous rupture of hepatocellular carcinoma with hemorrhagic shock: A multicenter study[J]. Springerplus, 2016, 5(1): 1101. DOI: 10.1186/s40064-016-2762-8. |
| [286] |
AOKI T, KOKUDO N, MATSUYAMA Y, et al. Prognostic impact of spontaneous tumor rupture in patients with hepatocellular carcinoma: An analysis of 1160 cases from a nationwide survey[J]. Ann Surg, 2014, 259(3): 532-542. DOI: 10.1097/SLA.0b013e31828846de. |
| [287] |
LAI EC, LAU WY. Spontaneous rupture of hepatocellular carcinoma: A systematic review[J]. Arch Surg, 2006, 141(2): 191-198. DOI: 10.1001/archsurg.141.2.191. |
| [288] |
SHIN BS, PARK MH, JEON GS. Outcome and prognostic factors of spontaneous ruptured hepatocellular carcinoma treated with transarterial embolization[J]. Acta Radiol, 2011, 52(3): 331-335. DOI: 10.1258/ar.2010.100369. |
| [289] |
ROUSSEL E, BUBENHEIM M, LE TREUT YP, et al. Peritoneal carcinomatosis risk and long-term survival following hepatectomy for spontaneous hepatocellular carcinoma rupture: Results of a multicenter French study (FRENCH-AFC)[J]. Ann Surg Oncol, 2020, 27(9): 3383-3392. DOI: 10.1245/s10434-020-08442-5. |
| [290] |
ZHOU J, HUANG A, YANG XR. Liquid biopsy and its potential for management of hepatocellular carcinoma[J]. J Gastrointest Cancer, 2016, 47(2): 157-167. DOI: 10.1007/s12029-016-9801-0. |
| [291] |
GUO W, SUN YF, SHEN MN, et al. Circulating tumor cells with stem-like phenotypes for diagnosis, prognosis, and therapeutic response evaluation in hepatocellular carcinoma[J]. Clin Cancer Res, 2018, 24(9): 2203-2213. DOI: 10.1158/1078-0432.CCR-17-1753. |
| [292] |
ZHOU Y, WANG B, WU J, et al. Association of preoperative EpCAM Circulating Tumor Cells and peripheral Treg cell levels with early recurrence of hepatocellular carcinoma following radical hepatic resection[J]. BMC Cancer, 2016, 16: 506. DOI: 10.1186/s12885-016-2526-4. |
| [293] |
SUN YF, XU Y, YANG XR, et al. Circulating stem cell-like epithelial cell adhesion molecule-positive tumor cells indicate poor prognosis of hepatocellular carcinoma after curative resection[J]. Hepatology, 2013, 57(4): 1458-1468. DOI: 10.1002/hep.26151. |
| [294] |
GUO W, YANG XR, SUN YF, et al. Clinical significance of EpCAM mRNA-positive circulating tumor cells in hepatocellular carcinoma by an optimized negative enrichment and qRT-PCR-based platform[J]. Clin Cancer Res, 2014, 20(18): 4794-4805. DOI: 10.1158/1078-0432.CCR-14-0251. |
| [295] |
SUN YF, GUO W, XU Y, et al. Circulating tumor cells from different vascular sites exhibit spatial heterogeneity in epithelial and mesenchymal composition and distinct clinical significance in hepatocellular carcinoma[J]. Clin Cancer Res, 2018, 24(3): 547-559. DOI: 10.1158/1078-0432.CCR-17-1063. |
| [296] |
QU C, WANG Y, WANG P, et al. Detection of early-stage hepatocellular carcinoma in asymptomatic HBsAg-seropositive individuals by liquid biopsy[J]. Proc Natl Acad Sci U S A, 2019, 116(13): 6308-6312. DOI: 10.1073/pnas.1819799116. |
| [297] |
HUANG A, ZHANG X, ZHOU SL, et al. Plasma circulating cell-free DNA integrity as a promising biomarker for diagnosis and surveillance in patients with hepatocellular carcinoma[J]. J Cancer, 2016, 7(13): 1798-1803. DOI: 10.7150/jca.15618. |
| [298] |
HUANG A, ZHAO X, YANG XR, et al. Circumventing intratumoral heterogeneity to identify potential therapeutic targets in hepatocellular carcinoma[J]. J Hepatol, 2017, 67(2): 293-301. DOI: 10.1016/j.jhep.2017.03.005. |
| [299] |
HUANG A, ZHANG X, ZHOU SL, et al. Detecting circulating tumor DNA in hepatocellular carcinoma patients using droplet digital PCR is feasible and reflects intratumoral heterogeneity[J]. J Cancer, 2016, 7(13): 1907-1914. DOI: 10.7150/jca.15823. |
| [300] |
CAI J, CHEN L, ZHANG Z, et al. Genome-wide mapping of 5-hydroxymethylcytosines in circulating cell-free DNA as a non-invasive approach for early detection of hepatocellular carcinoma[J]. Gut, 2019, 68(12): 2195-2205. DOI: 10.1136/gutjnl-2019-318882. |
| [301] |
LI W, ZHANG X, LU X, et al. 5-Hydroxymethylcytosine signatures in circulating cell-free DNA as diagnostic biomarkers for human cancers[J]. Cell Res, 2017, 27(10): 1243-1257. DOI: 10.1038/cr.2017.121. |
| [302] |
LLOVET JM, MONTAL R, SIA D, et al. Molecular therapies and precision medicine for hepatocellular carcinoma[J]. Nat Rev Clin Oncol, 2018, 15(10): 599-616. DOI: 10.1038/s41571-018-0073-4. |
| [303] |
GAO Q, ZHU H, DONG L, et al. Integrated proteogenomic characterization of HBV-related hepatocellular carcinoma[J]. Cell, 2019, 179(2): 561-577. DOI: 10.1016/j.cell.2019.08.052. |
| [304] |
JIANG Y, SUN A, ZHAO Y, et al. Proteomics identifies new therapeutic targets of early-stage hepatocellular carcinoma[J]. Nature, 2019, 567(7747): 257-261. DOI: 10.1038/s41586-019-0987-8. |
| [305] |
SHI J, LAI EC, LI N, et al. Surgical treatment of hepatocellular carcinoma with portal vein tumor thrombus[J]. Ann Surg Oncol, 2010, 17(8): 2073-2080. DOI: 10.1245/s10434-010-0940-4. |
| [306] |
Japan LCSGO. General rules for the clinical and pathological study of primary liver cancer[M]. Tokyo: Kanehara, 2003.
|
| [307] |
KONDO M, MORIMOTO M, KOBAYASHI S, et al. Randomized, phase Ⅱ trial of sequential hepatic arterial infusion chemotherapy and sorafenib versus sorafenib alone as initial therapy for advanced hepatocellular carcinoma: SCOOP-2 trial[J]. BMC Cancer, 2019, 19(1): 954. DOI: 10.1186/s12885-019-6198-8. |
| [308] |
KUDO M, UESHIMA K, YOKOSUKA O, et al. Sorafenib plus low-dose cisplatin and fluorouracil hepatic arterial infusion chemotherapy versus sorafenib alone in patients with advanced hepatocellular carcinoma (SILIUS): A randomised, open label, phase 3 trial[J]. Lancet Gastroenterol Hepatol, 2018, 3(6): 424-432. DOI: 10.1016/S2468-1253(18)30078-5. |
| [309] |
LYU N, LIN Y, KONG Y, et al. FOXAI: A phase Ⅱ trial evaluating the efficacy and safety of hepatic arterial infusion of oxaliplatin plus fluorouracil/leucovorin for advanced hepatocellular carcinoma[J]. Gut, 2018, 67(2): 395-396. DOI: 10.1136/gutjnl-2017-314138. |
| [310] |
WANG Q, XIA D, BAI W, et al. Development of a prognostic score for recommended TACE candidates with hepatocellular carcinoma: A multicentre observational study[J]. J Hepatol, 2019, 70(5): 893-903. DOI: 10.1016/j.jhep.2019.01.013. |
| [311] |
XU L, PENG ZW, CHEN MS, et al. Prognostic nomogram for patients with unresectable hepatocellular carcinoma after transcatheter arterial chemoembolization[J]. J Hepatol, 2015, 63(1): 122-130. DOI: 10.1016/j.jhep.2015.02.034. |
| [312] |
HAN G, BERHANE S, TOYODA H, et al. Prediction of survival among patients receiving transarterial chemoembolization for hepatocellular carcinoma: A response-based approach[J]. Hepatology, 2020, 72(1): 198-212. DOI: 10.1002/hep.31022. |
| [313] |
KUDO M, UESHIMA K, IKEDA M, et al. Randomised, multicentre prospective trial of transarterial chemoembolisation (TACE) plus sorafenib as compared with TACE alone in patients with hepatocellular carcinoma: TACTICS trial[J]. Gut, 2020, 69(8): 1492-1501. DOI: 10.1136/gutjnl-2019-318934. |
| [314] |
CHANG X, LU X, GUO J, et al. Interventional therapy combined with immune checkpoint inhibitors: Emerging opportunities for cancer treatment in the era of immunotherapy[J]. Cancer Treat Rev, 2019, 74: 49-60. DOI: 10.1016/j.ctrv.2018.08.006. |
| [315] |
MARKS LB, YORKE ED, JACKSON A, et al. Use of normal tissue complication probability models in the clinic[J]. Int J Radiat Oncol Biol Phys, 2010, 76(3 Suppl): s10-s19. DOI: 10.1016/j.ijrobp.2009.07.1754. |
| [316] |
PAN CC, KAVANAGH BD, DAWSON LA, et al. Radiation-associated liver injury[J]. Int J Radiat Oncol Biol Phys, 2010, 76(3 Suppl): s94-s100. DOI: 10.1016/j.ijrobp.2009.06.092. |
| [317] |
CHON YE, SEONG J, KIM BK, et al. Gastroduodenal complications after concurrent chemoradiation therapy in patients with hepatocellular carcinoma: Endoscopic findings and risk factors[J]. Int J Radiat Oncol Biol Phys, 2011, 81(5): 1343-1351. DOI: 10.1016/j.ijrobp.2010.07.1986. |
| [318] |
HANNA GG, MURRAY L, PATEL R, et al. UK consensus on normal tissue dose constraints for stereotactic radiotherapy[J]. Clin Oncol (R Coll Radiol), 2018, 30(1): 5-14. DOI: 10.1016/j.clon.2017.09.007. |
| [319] |
EL-KHOUEIRY AB, SANGRO B, YAU T, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): An open-label, non-comparative, phase 1/2 dose escalation and expansion trial[J]. Lancet, 2017, 389(10088): 2492-2502. DOI: 10.1016/S0140-6736(17)31046-2. |
| [320] |
SANGRO B, PARK JW, FINN RS. CheckMate 459: Long-term survival outcomes with nivolumab versus sorafenib as first-line treatment in patients with advanced hepatocellular carcinoma[C]. ESMO-GI, 2020.
|
| [321] |
FINN RS, RYOO BY, MERLE P, et al. Pembrolizumab as second-line therapy in patients with advanced hepatocellular carcinoma in KEYNOTE-240: A randomized, double-blind, phase Ⅲ trial[J]. J Clin Oncol, 2020, 38(3): 193-202. DOI: 10.1200/JCO.19.01307. |
| [322] |
|
| [323] |
ZHANG Y, XU J, SHEN J. Update on overall survival (OS) of RESCUE: An open-label, phase 2 trial of camrelizumab (C) in combination with apatinib (A) in patients with advanced hepatocellular carcinoma (HCC)[J]. J Clin Oncol, 2021, 39(15_suppl): abstr 4076. DOI: 10.1200/JCO.2021.39.15_suppl.4076 |
| [324] |
FINN RS, IKEDA M, ZHU AX, et al. Phase Ⅰb study of lenvatinib plus pembrolizumab in patients with unresectable hepatocellular carcinoma[J]. J Clin Oncol, 2020, 38(26): 2960-2970. DOI: 10.1200/JCO.20.00808. |
| [325] |
LLOVET J, FINN RS, IKEDA M, et al. A phase 1b trial of lenvatinib (LEN) plus pembrolizumab (PEMBRO) in unresectable hepatocellular carcinoma (uHCC): Updated results[J]. Ann Oncol, 2019, 30(suppl_5): v253-v324.
|
| [326] |
QIN S, CHEN Z, LIU Y, et al. A phase Ⅱ study of anti-PD-1 antibody camrelizumab plus FOLFOX4 or GEMOX systemic chemotherapy as first-line therapy for advanced hepatocellular carcinoma or biliary tract cancer[J]. J Clin Oncol, 2019, 37(15_suppl): abstr 4074. DOI: 10.1200/JCO.2019.37.15_suppl.4074 |
| [327] |
EL-KHOUEIRY AB, YAU T, KANG YK, et al. Nivolumab (NIVO) plus ipilimumab (IPI) combination therapy in patients (Pts) with advanced hepatocellular carcinoma (aHCC): Long-term results from CheckMate 040[J]. J Clin Oncol, 2021, 39(3_suppl): abstr 269.
|
| [328] |
KELLEY RK, SANGRO B, HARRIS WP, et al. Efficacy, tolerability, and biologic activity of a novel regimen of tremelimumab (T) in combination with durvalumab (D) for patients (pts) with advanced hepatocellular carcinoma (aHCC)[J]. J Clin Oncol, 2020, 38(15_suppl): abstr 4508. DOI: 10.1200/JCO.2020.38.15_suppl.4508 |
| [329] |
HAN C, YE S, HU C, et al. Clinical activity and safety of penpulimab (Anti-PD-1) with anlotinib as first-line therapy for unresectable hepatocellular carcinoma: An open-label, multicenter, phase Ⅰb/Ⅱ trial (AK105-203)[J]. Front Oncol, 2021, 11: 684867. DOI: 10.3389/fonc.2021.684867. |




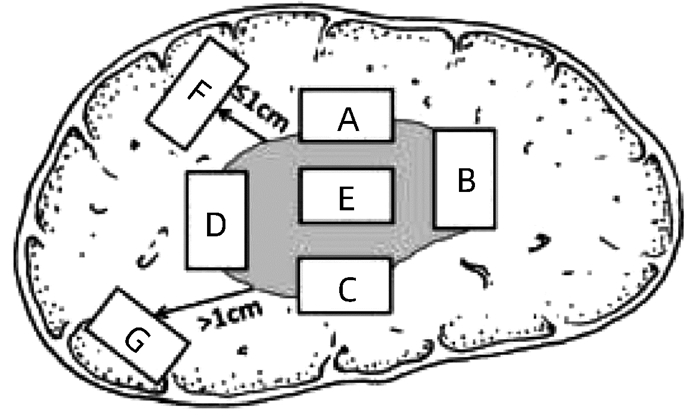




 DownLoad:
DownLoad: