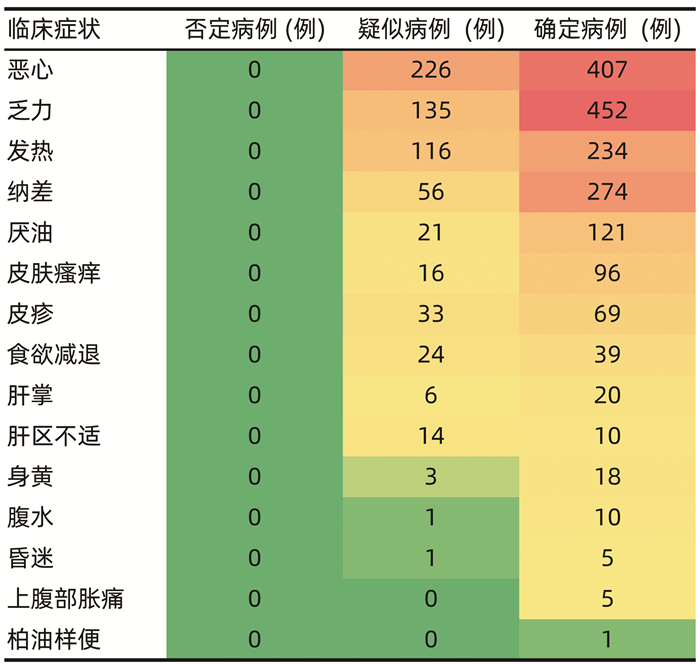
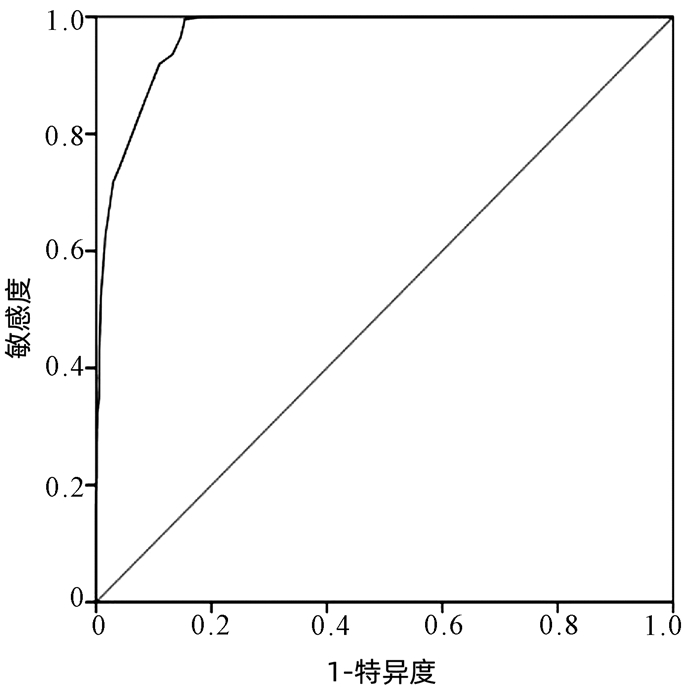
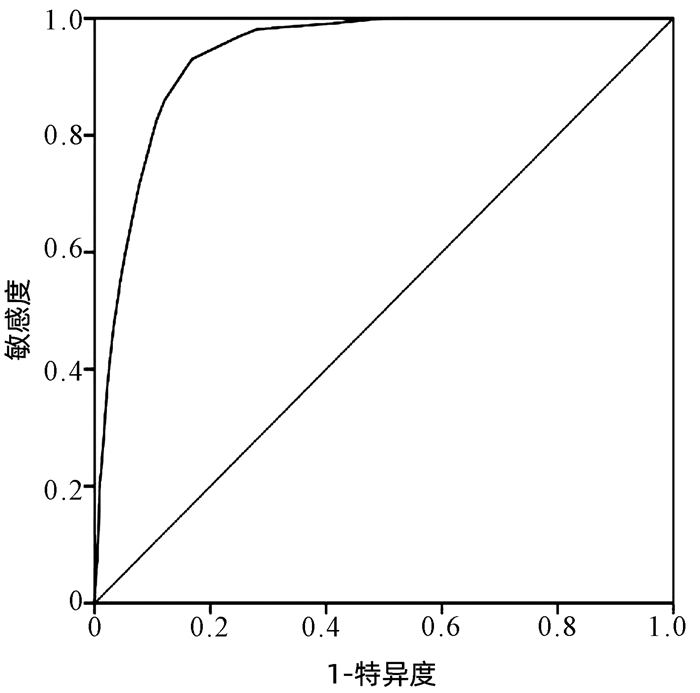
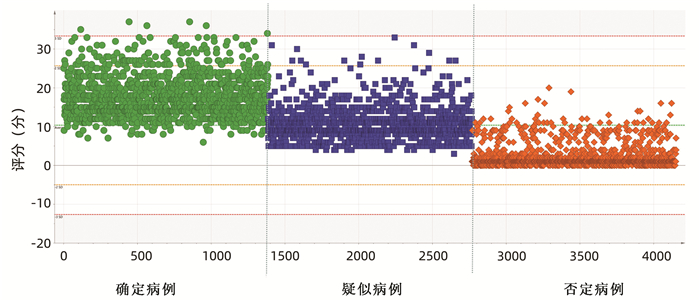
| [1] |
|
| [2] |
|
| [3] |
Drug-Induced Liver Disease Study Group, Chinese Society of Hepatology, Chinese Medical Association. Guidelines for the management of drug-induced liver injury[J]. J Clin Hepatol, 2015, 31(11): 1752-1769. DOI: 10.3969/j.Issn.1001-5256.2015.11.002.
|
| [4] |
GE FL, NIU M, HAN ZX, et al. Landscape of hepatobiliary adverse drug reactions related to preparations containing psoraleae fructus and its application in pharmacovigilance[J]. Chin J Integr Med, 2021, 27(11): 832-837. DOI: 10.1007/s11655-021-3442-2.[Online ahead of print] |
| [5] |
GE FL, GUO YM, CAO JL, et al. Research progress on evaluation methods and risk factors for Chinese medicines-induced liver injury[J]. Mod Chin Med, 2019, 21(3): 284-290. DOI: 10.13313/j.issn.1673-4890.20180925002. |
| [6] |
DU XX, SONG HB, REN JT, et al. Opportunities and challenges of post-marketing evaluation of raditional Chinese medicine[J]. Chin J Chin Mater Med, 2014, 39(18): 3427-3429. DOI: 10.4268/cjcmm20141803. |
| [7] |
MAO YM. Strengthening the scientific research and supervision of drug-induced liver injury based on big data[J]. J Clin Hepatol, 2018, 34(6): 1166 -1168. DOI: 10.3969/j.issn.1001-5256.2018.06.005. |
| [8] |
State Drug Administration. Technical guidelines for clinical evaluation of liver injury induced by traditional Chinese medicine[J]. J Clin Hepatol, 2018, 34(7): 1403-1409. DOI: 10.3969/j.issn.1001-5256.2018.07.008. |
| [9] |
|
| [10] |
PAN XC. Drug risk assessment based on cluster analysis[D]. Nanjing: Nanjing University of Posts and Telecommunications, 2020.
潘轩超. 基于聚类分析的药物风险评估[D]. 南京: 南京邮电大学, 2020.
|
| [11] |
ZHU Q, DING J, REN WX, et al. Study on risk assessment model of in vitro diagnostic reagent adverse events based on BP neural network[J]. Chin J Med Instrument, 2019, 43(2): 136-139. DOI: 10.3969/j.issn.1671-7104.2019.02.017. |
| [12] |
HARPAZ R, DUMOCHEL W, SHAH NH. Big data and adverse drug reaction detection[J]. Clin Pharmacol Ther, 2016, 99(3): 268-270. DOI: 10.1002/cpt.302. |
| [13] |
MA HL, XU CY, YANG XM. Application status of decision tree in traditional Chinese medicine[J]. World Chin Med, 2021, 16(17): 2648-2651, 2656. DOI: 10.3969/j.issn.1673-7202.2021.17.025. |
| [14] |
GE FL, NIU M, HAN ZX, et al. Analysis of epidemiological characteristics of drug induced liver injury associated with baixianpi preparations[J]. Chin J Chin Mater Med, 2019, 44(5): 1048-1052. DOI: 10.19540/j.cnki.cjcmm.20181217.001. |



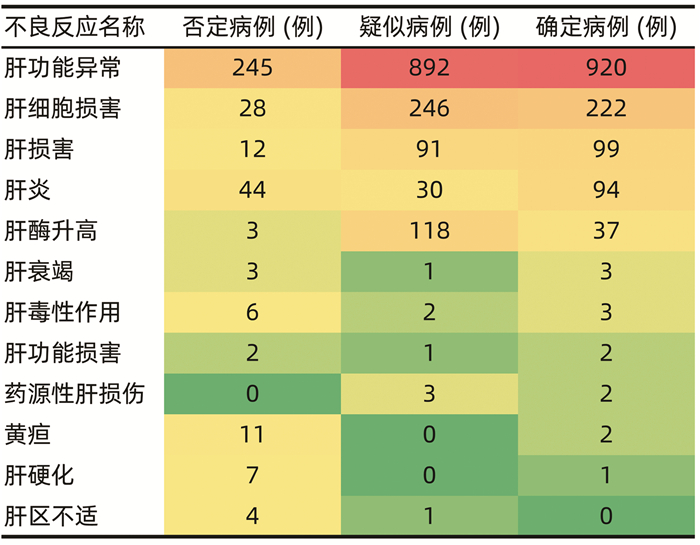




 DownLoad:
DownLoad: